
Dr. Fernández is a Clinical Professor of Surgery and Family Practice at the University of Texas Health Center at Tyler. Dr. Fernández obtained his residency training at Loyola University, the University of Illinois, the University of Chicago and Northwestern University, Chicago, Illinois, with special training in trauma and burns at Kings County Hospital, SUNY Down State System, Brooklyn, New York.
He is well published in multiple areas of research both in peer-reviewed articles and book chapters, and has made numerous oral and poster presentations at national and international conferences. Dr. Fernández is a paid consultant of 3M.
Fernandez_Current-Dialogues-in-Wound-Management_2021_Article-18
Skin injury related to medical adhesive use in negative pressure wound therapy (NPWT) is an often underrecognized complication that impacts all care settings and can affect all patient groups to varying degrees.1 The use of proper technique for application and removal of adhesive products is essential to diminish the risk of tissue trauma. Failure to recognize skin injury from medical adhesive use can occur, impacting patient safety and quality of life and increasing healthcare costs.2
EFFECTS OF MEDICAL ADHESIVE DRESSINGS ON SKIN:
Medical adhesive-related skin injuries (MARSI) occur when the attachment forces between the skin and a medical adhesive are stronger than those between the individual skin cells. This causes the separation of the epidermal layers which may lead the epidermis to completely detach from the dermis.3
MARSI:
Chronic and acute complex wounds in topographically difficult anatomical locations are challenging. These types of wounds will test the most experienced clinician and require the use of wound care techniques that may increase a patient’s risk of developing medical adhesive-related skin injury (MARSI).3
MARSI DEFINITION:
In a recent 2020 consensus statement Fumarola et al defined medical adhesive-related skin injury (MARSI) as:3
“Skin damage related to the use of medical adhesive products or devices such as tapes, wound dressings, stoma products, electrodes, medication patches and wound closure strips.”
Dermal injuries associated with the use of medical adhesives have three main underlying contributing factors: mechanical shear forces (blistering, skin tears), chemical irritation dermatitis due to the adhesive agent, and maceration of the skin and folliculitis due to prolonged occlusive dressing’s detrimental effect on the skins’ inability to remove excess local fluid or moisture (Table 1).
TABLE 1. IDENTIFYING MARSI
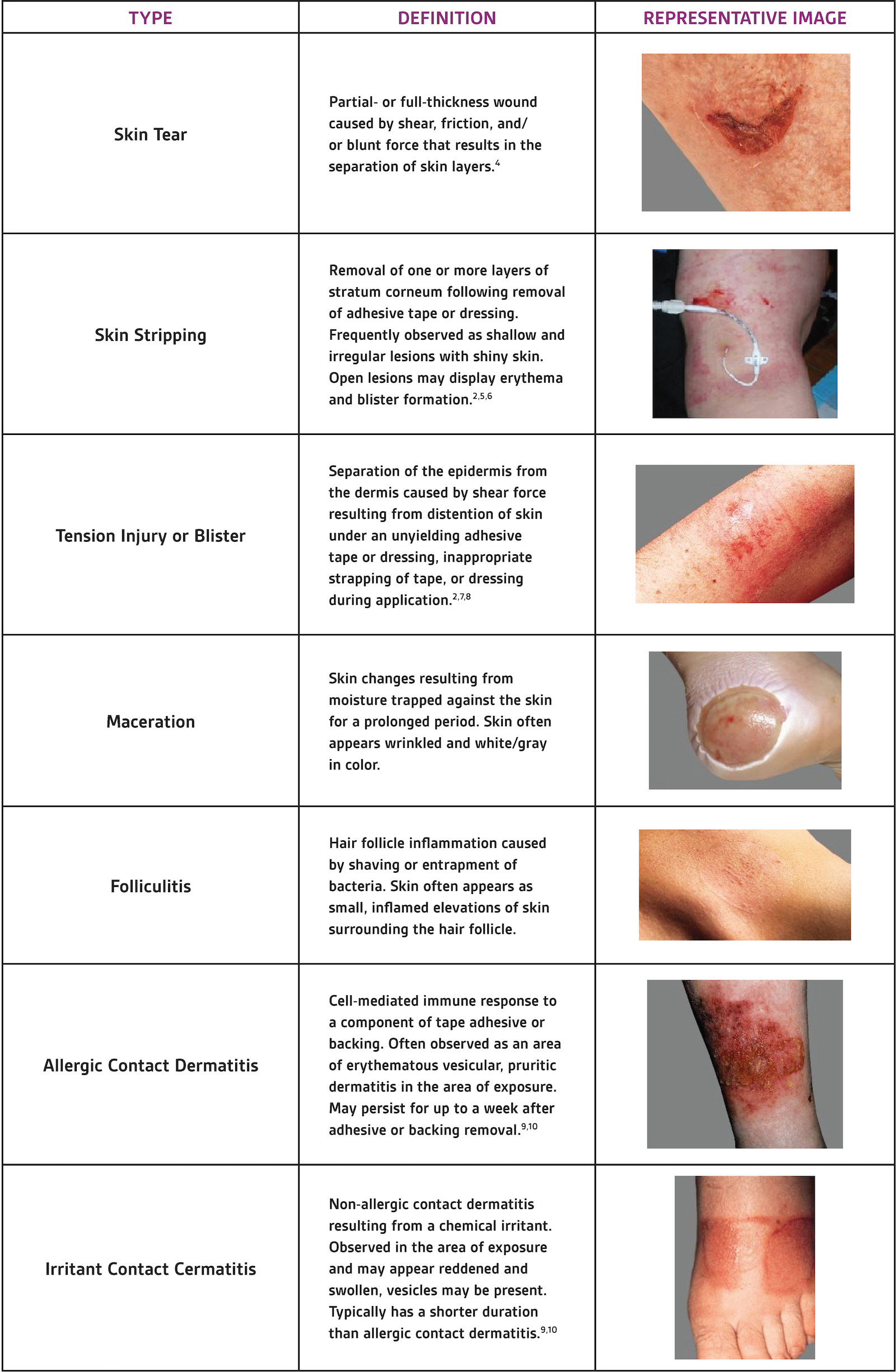
INCIDENCE:
Several studies have attempted to determine the incidence of MARSI. Results vary and are, in part, related to the patient’s comorbidities, age and clinical condition. In a random sample of MARSI studies, the lowest incidence of MARSI recorded was 5.8% for outpatients from an academic medical center,3 and in a recent U.S. study it was approximately 13% .9,11
PREVENTION:
In December 2012 a consensus panel of 23 clinicians, funded through an unrestricted educational grant from 3M, was convened to help define best practices recommendations for assessment, prevention, and treatment of these types of injuries. In 2013 McNichol et al published a comprehensive review of the consensus panel’s recommendations.9
Some of the key clinical takeaways from this review include:

BIOMECHANICS OF COMMON ADHESIVES:
Acrylate adhesives are the most common adhesive used for patient care. They provide a highly effective contact interface with the underlying skin. Because of their strong bond to the epidermis, they may increase the risk of pain upon removal and have been associated with MARSI.1-3,9,11 Silicone adhesives are increasingly used because they are gentler to the underlying skin (Figure 1).12
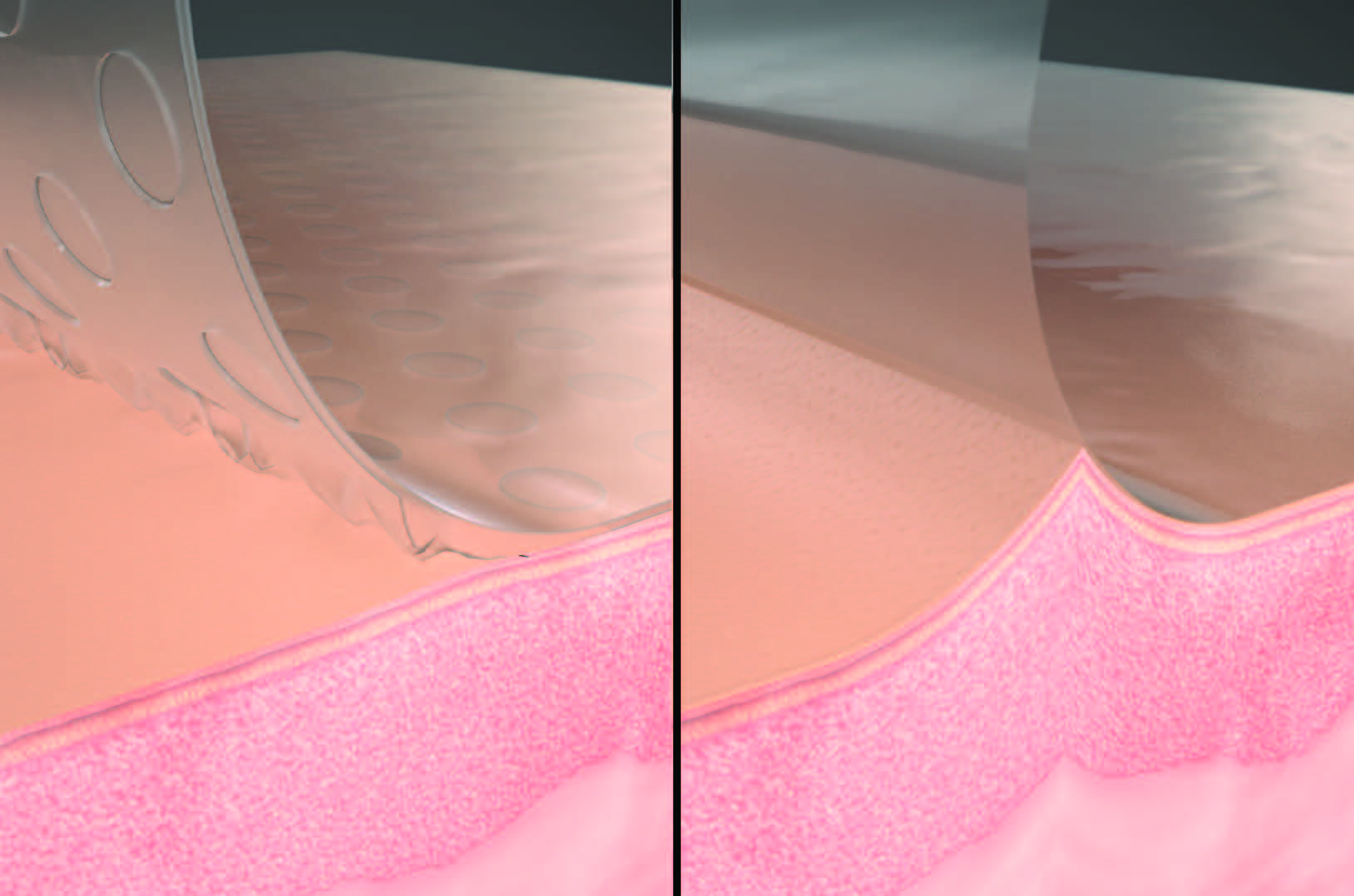
3M™ DERMATAC™ DRAPE:
NPWT is commonly used to manage complex wounds and has traditionally required the use of a polyurethane drape with an acrylic adhesive over foam dressings to create a negative pressure seal.12-15 This traditional drape provides excellent adhesion; however, it has some well described drawbacks. Once applied, the drape cannot be easily repositioned. Inappropriate handling has been associated with MARSI, skin blistering, and drape removal during dressing changes can be painful.1-3,9,11,16 A novel hybrid drape with acrylic adhesive and a silicone perforated layer has been developed for use with NPWT.
The Dermatac Drape combines the benefits of both silicone and acrylic adhesive for use in conjunction with NPWT (Figure 2). This adhesive drape can be easily modified, detaches without difficulty, and can be repositioned at initial application.17 These features help to decrease the time associated with with drape application for dressing applications and changes compared to standard acrylic adhesive dressings. The Dermatac Drape creates a fully occlusive seal that will last up to 72 hours while acting as a barrier to external contaminants and helps provide peri-wound protection.18 It conforms to a variety of anatomical locations and helps provide an effective seal in uneven wounds. The silicone component in the Dermatac Drape allows for skin-friendly removal and improves patient comfort during application and removal of the drape.17 The novel hybrid drape has been used with 3M™ V.A.C.® Therapy and 3M™ Veraflo™ Therapy (Figure 3).

APPLICATION OF
DERMATAC™ DRAPE:
The Dermatac Drape is intended for use in conjunction with the following NPWT systems:
• 3M™ ActiV.A.C.™ Therapy System
• 3M™ V.A.C.® Simplicity Therapy System
• 3M™ V.A.C.® Via Therapy System
• V.A.C. FREEDOM™ Therapy System
• 3M™ V.A.C.® Ulta Therapy System
• 3M™ V.A.C.® RX4 Therapy System
The ActiV.A.C. Therapy, V.A.C.® Simplicity Therapy, V.A.C.® Via Therapy, and V.A.C. Freedom systems are wound management systems commonly used in the acute, extended and home therapy environment. While the V.A.C.® Ulta Therapy and V.A.C.® RX4 Therapy systems, which are integrated wound management systems, are used in acute care settings and other professional healthcare environments where product use is conducted by or under the supervision of a qualified healthcare professional.
Dermatac Drape application differs from the traditional acrylic adhesive drape application due to the differences in the adhesive components of the drape.
The application steps for Dermatac Drape are as follows:
1. Following the instructions for use, place the appropriate dressing into the wound bed.
2. Trim the Dermatac Drape to cover the dressing and 5-7 cm of intact periwound skin.
a. There is no need to cut the drape into multiple strips or to employee the window frame technique as is commonly done with traditional acrylic drape.
3. Remove the release liner and hold the drape by the handling bars.
4. Place the adhesive side face down over the dressing and periwound skin, ensuring the drape covers at least 5-7 cm of intact periwound skin.
a. Make sure to avoid pulling or stretching of the drape over the dressing to avoid potential periwound skin trauma.
5. Holding down the edge of the drape, remove the perforated handling bars followed by patting down the drape to ensure an occlusive seal.
6. Smooth any wrinkles or creases in drape to prevent leaks.
a. The drape can be peeled back and re-applied during initial placement, if necessary.
b. There is no need for a skin barrier, as the Dermatac Drape can conform to a wide variety of anatomical locations.
7. Once Dermatac Drape has been placed, follow the instructions for use on how to apply the connecting tubing and initiating negative pressure or instillation therapy for V.A.C.® Therapy or Veraflo™ Therapy.
CONCLUSION
Complex wounds in difficult anatomical locations are challenging and require the use of wound care techniques that may increase a patient’s risk of developing MARSI.3 Clinicians can help minimize the risk of MARSI by using the most appropriate adhesive products and proper application techniques.9 While acrylate adhesives are the most common adhesive used in patient care, their use has been associated with MARSI.1-3,9,11 New developments in medical adhesives have led to the increased use of silicone adhesive as they are gentler to the underlying skin.12
Dermatac Drape combines the benefits of both silicone and acrylic adhesive for use in conjunction with negative pressure therapy. This adhesive drape can be easily modified, detaches from skin without difficulty, and can be repositioned at initial application.17 The Dermatac Drape creates a fully occlusive seal, acts as a barrier to external contaminants,18 helps provide peri-wound protection, and conforms to a variety of anatomical locations. There is preliminary clinical experience that shows that Dermatac Drape has been used in challenging wounds and has demonstrated sealing capabilities that are effective and comparable to the traditional acrylic drape.19 With its low tack adhesive properties, the Dermatac Drape has been shown to be strong enough to maintain a seal for both V.A.C.® Therapy and Veraflo™ Therapy.19-21
The application of Dermatac Drape is simple and straight forward; however, clinicians should carefully review the application instructions prior to drape placement due to the differences in application between traditional drape and the Dermatac Drape. Specifically, the Dermatac Drape should be trimmed to cover the dressing and 5-7 cm of intact periwound skin. The drape should not be cut into strips as this will reduce its effectiveness.
References
References
- Collier M. Minimising pain and medical adhesive related skin injuries in vulnerable patients. Br J Nurs. 2019;28(15):S26-S32.
- Farris MK, Petty M, Hamilton J, Walters SA, Flynn MA. Medical Adhesive-Related Skin Injury Prevalence Among Adult Acute Care Patients: A Single-Center Observational Study. J Wound Ostomy Continence Nurs. 2015;42(6):589-598.
- Fumarola S, Allaway R, Callaghan R, et al. Overlooked and underestimated: medical adhesive-related skin injuries. J Wound Care. 2020;29(Sup3c):S1-S24.
- Lund CH, Tucker JA. Adhesion and Newborn Skin. In: Hoath SB, Maibach HI, eds. Neonatal Skin: Structure and Function. 2nd ed. Boca Raton, FL: Taylor & Francis Group; 2003:299-324.
- Resnick B. Wound care for the elderly. Each type of wound care product has advantages and disadvantages. Geriatr Nurs. 1993;14(1):26-29.
- Smith MA, Jones NMM, Page SL, Peck Dirda M. Pressure-sensitive tape and techniques for its removal from paper. J Am Inst Conserv. 1984;23(2):101-113.
- Gerhardt LC, Lenz A, Spencer ND, Munzer T, Derler S. Skin-textile friction and skin elasticity in young and aged persons. Skin Res Technol. 2009;15(3):288-298.
- Shannon ML, Lehman CA. Protecting the skin of the elderly patient in the intensive care unit. Crit Care Nurs Clin North Am. 1996;8(1):17-28.
- McNichol L, Lund C, Rosen T, Gray M. Medical adhesives and patient safety: state of the science: consensus statements for the assessment, prevention, and treatment of adhesive-related skin injuries. J Wound Ostomy Continence Nurs. 2013;40(4):365-380.
- Lund C. Medical Adhesives in the NICU. Newborn Infant Nurs Rev. 2014;14(4):160-165.
- Ratliff CR. Descriptive study of the frequency of medical adhesive-related skin injuries in a vascular clinic. J Vasc Nurs. 2017;35(2):86-89.
- Schalau GK, II, Bobenrieth A, Huber RO, Nartker LS, Thomas X. Silicone Adhesives in Medical Applications. In: Ozer H, ed. Applied Adhesive Bonding in Science and Technology. IntechOpen; 2021:93-117.
- Vuerstaek JD, Vainas T, Wuite J, Nelemans P, Neumann MH, Veraart JC. State-of-the-art treatment of chronic leg ulcers: A randomized controlled trial comparing vacuum-assisted closure (V.A.C.) with modern wound dressings. J Vasc Surg. 2006;44(5):1029-1038.
- Blume PA. Comparison of Negative Pressure Wound Therapy Using Vacuum-Assisted Closure With Advanced Moist Wound Therapy in the Treatment of Diabetic Foot Ulcers: a Multicenter Randomized.
- de Laat EH, van den Boogaard M, Spauwen PH, van Kuppevelt DH, van Goor H, Schoonhoven L. Faster wound healing with topical negative pressure therapy in difficult-to-heal wounds: A prospective randomized controlled trial. Ann Plast Surg. 2011;67(6):626-631.
- Monsen C, Acosta S, Mani K, Wann-Hansson C. A randomised study of NPWT closure versus alginate dressings in peri-vascular groin infections: quality of life, pain and cost. J Wound Care. 2015;24(6):252-260.
- Jester R, Russell L, Fell S, Williams S, Prest C. A one hospital study of the effect of wound dressings and other related factors on skin blistering following total hip and knee arthroplasty. J Orthop Nurs. 2000;4(2):71-77.
- KCI. Dermatac™ PU layer as a bacterial barier. June 7, 2016. 2016.00000437077.REF-01641.
- Fernandez LG, Matthews MR, Benton C, et al. Use of a novel silicone-acrylic drape with negative pressure wound therapy in anatomically challenging wounds. Int Wound J. 2020;17(6):1829-1834.
- Matthews MR, Fernandez L. Applications of a New Silicone-Acrylic Hybrid Semiocclusive Drape With Negative Pressure Wound Therapies in a Burn Center. Wounds. 2021.
- Greenstein E, Moore N. Use of a Novel Silicone-Acrylic Drape With Negative Pressure Wound Therapy in Four Patients With Periwound Skin Breakdown. Wounds. 2021.
Patient images courtesy of Luis G. Fernández, MD, KHS, KCOEG, FACS, FASAS, FCCP, FCCM, FICS.
NOTE: Specific indications, contraindications warnings, precautions, and safety information exist for these products and therapies. Please consult a clinician and product instructions for use prior to application. Rx only.
©2022 3M. All rights reserved. 3M and the other marks shown are marks and/or registered marks. Unauthorized use prohibited. Used under license in Canada. All other marks are property of their respective owners.


