
Dr. Jonathan Arnold obtained his medical degree in 1997 from the University of Oklahoma College of Medicine in Oklahoma City. He is board certified by the American Board of Family Medicine, the American Board of Preventive Medicine – Undersea and Hyperbaric Medicine (ABPM-UHM). He is also credentialed by the American Board of Wound Management as a Certified Wound Specialist Physician (CWS-P). Dr. Arnold is a paid consultant for 3M.
Arnold_Current-Dialogues-in-Wound-Management_2023_Article-6
With decades of evidence supporting its use, negative pressure wound therapy (NPWT) remains a key method of managing problem wounds. NPWT is also a therapy of choice in aggressive front-line wound management when patients present with multiple risk factors such as poorly controlled diabetes and/or renal failure.1,2 Furthermore, recent design advancements have improved the portability of NPWT while continuing to deliver effective negative pressure to the wound area.3-6 More portable NPWT systems, such as 3M™ Snap™ Therapy System, are dis-posable, smaller in size, light weight, allows patients to ambulate (depending on wound location) and easily conceal the device under clothing. Snap Therapy utilizes mechanical power to deliver -125 mmHg, eliminating the need for electrical power during use and reducing the noise during operation, opening up the option for increased patient mobility. These features have made Snap Therapy more tolerable for patients. Exit surveys indicated patients receiving Snap Therapy reported reduced therapy noise levels, interruption of daily activities and sleep, and impact on social situations compared to patients receiving traditional NPWT.5 While Snap Therapy can provide another option for NPWT, it is not recommended for every wound. Wounds that are larger than 13 x 13 cm2, producing more than 180 mL of exudate a week, have active bleeding, malignancy, untreated infection/osteomyelitis, or necrotic tissue should not be managed with Snap Therapy. Three cases are presented below illustrating the effective use of Snap Therapy in surgical wounds.
CASE 1
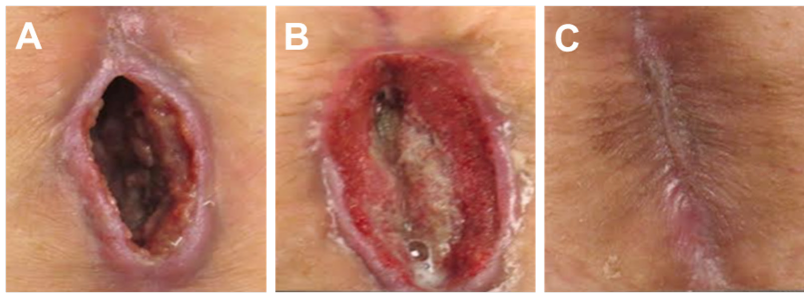
A 76-year-old female presented for care with a two-month history of a non-healing wound following an exploratory laparotomy. The wound surface area at presentation was 4.2 cm2 and the wound volume was 9.3 cm3 (Figure 1A). The wound was also noted to have a 5.5 cm tunnel extending proximally from the wound base toward the umbilicus. Excisional debridement was performed prior to the initial application of Snap Therapy. Dressing changes occurred twice a week. The proximal 5.5 cm tunnelling lesion was resolved at the first dressing change on Day 3 (Figure 1B). Excisional debridement was again performed on Days 7 and 14 of treatment. No other adjunctive or advanced wound care modalities were utilized. A total of six applications of Snap Therapy were applied over 3.5 weeks, resulting in complete wound closure with minimal scar (Figure 1C).
CASE 2
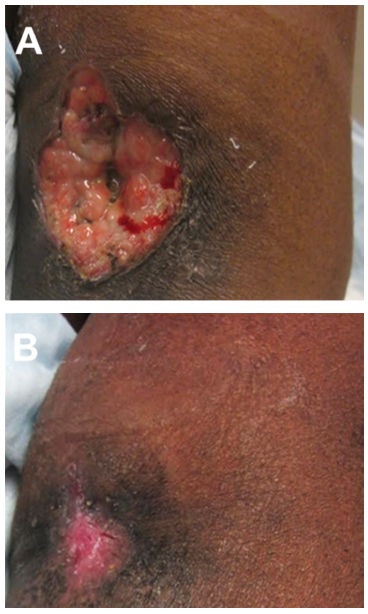
A 59-year-old female with end-stage renal disease (ESRD) on hemodialysis was referred for wound management following incision and drainage of a left antecubital fossa abscess. The abscess extended to the left arteriovenous (AV) fistula dialysis access site. The wound area at presentation was 12.5 cm2 and the wound volume measured 11.2 cm3 (Figure 2A). Sharp debridement was performed prior to the initial application of Snap Therapy. Dressing changes occurred twice a week. The wound was debrided a second time on Day 14 of treatment. No adjunctive or other advanced wound care modalities were utilized. The wound required six applications of Snap Therapy over five weeks, resulting in rapid reduction in wound volumes and complete wound closure (Figure 2B). There was no noted damage to the underlying AV fistula dialysis access site.
CASE 3
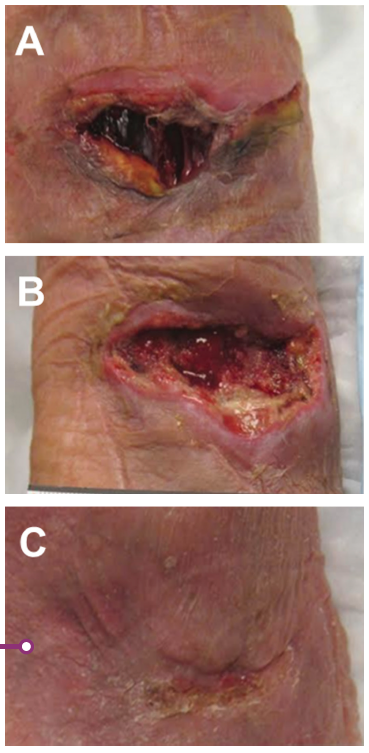
A 78-year-old female presented for further evaluation and treatment of right antecubital fossa abscess complicated by methicillin-resistant Staphylococcus aureus (MRSA). The wound was initially evaluated in the hospital emergency department and treated with incision and drainage. Antibiotics were prescribed and the wound culture result confirmed appropriate antibiotic coverage. The wound volume at presentation was 7.4 cm2 and the wound volume was 5.9 cm3 (Figure 3A). Excisional debridement was performed prior to the initial application of Snap Therapy (Figure 3B). Dressing changes occurred twice per week. Excisional debridement was performed again at day 14 of treatment. No other adjunctive or advanced wound care modalities were utilized. A total of five applications of Snap Therapy over four weeks resulted in rapid reduction in wound volume with wound closure obtained in four weeks (Figure 3C).
CONCLUSION
Conventional electrical NPWT revolutionized the treatment of large, complex acute and chronic wounds. However, its requirement for access to electrical power, relatively large size, and occasional problems with pump noise and collection canister odor represent significant barriers to effective wound care. Furthermore, the time required for application and the procurement process can be challenginvg to both the patient and care provider.
Snap Therapy addresses smaller, complex wounds in the outpatient setting. It does not require electrical power, is disposable, and is ready to use “off-the-shelf”. Its small size, quiet operation and portable configuration have been shown to provide similar wound healing rates when compared to conventional electrically powered NPWT.6 Moreover, the improved patient quality of life indicators have been noted when comparing Snap Therapy to conventional electrically powered NPWT.5 In these three patients, use of Snap Therapy resulted in wound healing within 5 weeks from presentation.
References
- De Caridi G, Serra R, Massara M, et al. VAC therapy for the treatment of complex wounds after cardio-thoracic surgery. Int Wound J. 2016;13(5):759-762. doi:10.1111/iwj.12369
- James SM, Sureshkumar S, Elamurugan TP, Debasis N, Vijayakumar C, Palanivel C. Comparison of Vacuum-Assisted Closure Therapy and Conventional Dressing on Wound Healing in Patients with Diabetic Foot Ulcer: A Randomized Controlled Trial. Nigerian Journal of Surgery. 2019;25(1):14-20. doi:10.4103/njs.NJS_14_18
- Fong KD, Marston WA. SNaP Wound Care System: Ultraportable Mechanically Powered Negative Pressure Wound Therapy. Adv Wound Care. 2012;1(1):41-43. doi:10.1089/wound.2011.0281
- Lerman B, Oldenbrook L, Ryu J, Fong KD, Schubart PJ. The SNaP wound care system: A case series using a novel ultraportable negative pressure wound therapy device for the treatment of diabetic lower extremity wounds. J Diabetes Sci Technol. 2010;4(4):825-830.
- Armstrong DG, Marston WA, Reyzelman AM, Kirsner RS. Comparative effectiveness of mechanically and electrically powered negative pressure wound therapy devices: A multicenter randomized controlled trial. Wound Repair Regen. 2012;20(3):332-341.
- Armstrong DG, Marston WA, Reyzelman AM, Kirsner RS. Comparison of negative pressure wound therapy with an ultraportable mechanically powered device vs. traditional electrically powered device for the treatment of chronic lower extremity ulcers: A multicenter randomized-controlled trial. Wound Repair Regen. 2011;19(2):173-180.
NOTE: Specific indications, contraindications, warnings, precautions, and safety information exist for these products and therapies. Please consult a clinician and product Instructions for Use prior to application. Rx only.
As with any case study, the results and outcomes should not be interpreted as a guarantee or warranty of similar results. Individual results may vary depending on the patient’s circumstances and condition.
Patient data and images courtesy of Jonathan Arnold, MD, CWS-P.
© 2023 3M. All rights reserved. 3M and the other marks shown are marks and/or registered marks. Unauthorized use prohibited. Used under license in Canada.

