
Dr. Jeffrey Niezgoda is the President & Chief Medical Officer of AZH Centers. Advancing the Zenith of Healthcare is a novel company dedicated to excellence in wound, vascular, and regenerative clinical services. He is the Vice President of the American Professional Wound Care Association and the Immediate Past President of the American College of Hyperbaric Medicine. He has participated in multiple Expert Consensus Panels and has appeared before Medicare and Medicaid Services and the Food and Drug Administration to provide expert opinion in wound care and hyperbaric medicine. Dr. Niezgoda holds an M.D. from the Uniformed Services University of the Health Sciences, F. Edward Hébert School of Medicine, Bethesda, Maryland, and is a 1981 graduate of the U.S. Air Force Academy.
Niezgoda_Current Dialogues in Wound Management_2018_Volume 4_Issue 3
NOTE: As with any case study, the results and outcomes should not be interpreted as a guarantee or warranty of similar results. Individual results may vary depending on the patient’s circumstances and condition.
Wound healing is a tightly controlled set of cellular and physiological processes that progress through hemostasis, inflammation, proliferation, and tissue remodeling.1 However, in stalled or non-healing wounds, the transition from the inflammatory phase to the proliferative phase is commonly disrupted, often with detrimental results. While a number of factors can cause wound healing to stall, wound infection or chronic inflammation are common culprits that can be difficult to identify correctly.
WOUND BIOBURDEN AND INFECTION
The presence and level of bacteria within wounds is well known to affect wound healing. Bacterial contamination occurs when non-replicating bacteria are present in the wound without altering wound healing. Bacterial colonization occurs when wound bacteria begin replicating but do not affect wound healing.2As microbial virulence and/or quantity increases, wound contamination increases, leading to critical colonization which may progress to local infection (a bacterial burden of >105 organisms per gram of tissue that elicits a host response).3 During wound infection, the invading microbe is in direct competition with the host cells for substrates necessary for cell proliferation, altering the host’s ability to undergo the wound healing process.4 If left unchecked or treated improperly, local infection can progress to systemic infection which may lead to organ failure, sepsis, septic shock, and in some cases, death.
Bacteria can also aggregate together as biofilm and secrete an extracellular polysaccharide matrix (EPS) to protect themselves from host immune response and antibiotic treatments, and aid in adhesion to surfaces and other bacteria.5,6 The slow growth of biofilm bacteria, physical barrier provided by the EPS, and EPS macromolecules binding to topical antimicrobial treatments contribute to biofilm resistance.7 Additionally, biofilms can rapidly re-develop following debridement,7further complicating wound treatment.
Outside of laboratory diagnosis, clinical signs of infection exist; although not all patients will display clinical manifestations of infection despite having abnormal laboratory results. Clinical signs and symptoms of wound infection include pain, local warmth, malodor, erythema, persisting or increasing exudate, and cellulitis. The tissue may show ischemia, necrosis, and absent or poor-quality granulation consistent with wound bed deterioration.
WOUND INFLAMMATION
At the cellular and molecular level, disruption of the protective dermis allows for potential bacterial contamination. In response, leukocytes (such as macrophages, neutrophils, and mast cells) are recruited to the injury site as part of the inflammatory response to remove potential pathogens and cell debris.8,9 Neutrophils and macrophages are recruited to the site of injury through the secretion of the host’s defense molecules including antimicrobial peptides, chemokines, and cytokines.9 The recruited leukocytes then secrete cytokines, and proteinases to kill and remove pathogens, and drive further recruitment of leukocytes to the injury site.9,10Phagocytosis of the invading microbes releases reactive oxygen species (ROS) to directly attack and disrupt bacterial cell walls, and help coordinate the increased recruitment of immune cells to the wound.8,11 A delicate balance exists as an excessive amount of ROS can lead to host cell damage, overzealous recruitment of immune cells to the injury site, and stalled wound healing.
One notable feature of chronic inflammationis the continued and excessive production of ROS and matrix metalloproteinases (MMPs).7,12Additionally, ROS, MMPs, elastases, and other proteases can contribute to oxidative stress through the degradation of growth factors and components of the extracellular matrix, resulting in the continuation of the inflammation response at the expense of wound healing.
Clinical symptoms of wound inflammation include pain, local periwound erythema, and persisting or increasing exudate. The wound bed tissue typically shows absent or poor-quality granulation tissue and slow or no epithelialization.
MANAGEMENT
It can be difficult to discern the difference between wound infection and chronic inflammation. Initially, it is better to err on the side of caution and treat for wound infection. However, chronic inflammation should be considered when management of suspected infection fails to demonstrate wound improvement and healing appears stalled. Antibiotic options for wound infection treatment are varied, and the choice between topical or parenteral antibiotics should be based on the signs of infection. Topical antibiotics can be considered when local infection is suspected with no significant tissue extension. Parenteral antibiotics should be considered when then the signs and symptoms of infection extend beyond the local wound and into deep tissues or if systemic signs of infection are present.
Debridement should be considered in the management of stalled wounds. Removal of devitalized tissue, slough, and infectious materials can help transform the chronic wound bed and reinitiate acute phase healing. Repeated sharp debridement followed by wound cleansing with topical antiseptics and appropriate infection treatment can help to mitigate biofilm development and control infection.
Management of ROS and MMPs may also help stimulate healing in wounds stalled by chronic inflammation. Elevated levels of ROS have been associated with impaired wound healing in chronic wounds.15 Additionally, high levels of MMPs have been reported to correlate with the severity of ulcers.16 Thus, lowering the levels of ROS and MMPs may help wound healing progress through the inflammation phase and into the proliferation phase. An in vitro study has reported a reduction in protease (MMP and elastase) levels in diabetic foot ulcer wound exudate following the application of collagen and oxidized regenerated cellulose (ORC).
CLINICAL CASE STUDY
A 56-year-old male with insulin-dependent diabetes mellitus sustained a traumatic injury to his left ankle. Initial management included negative pressure wound therapy (NPWT) following debridement. NPWT was continued for approximately 4 weeks, at which time the wound base was granulated and without significant depth or undermining. Serial bi-weekly follow-up visits demonstrated periwound erythema, slight tenderness, mild odor, and moderate exudate. The patient was started on topical antimicrobial therapy followed by a 10-day course of oral doxycycline (100mg). After 4 weeks of antimicrobial therapy the appearance of the wound was essentially unchanged (Figure 1).
 Figure 1. Wound after 4 weeks of antimicrobial therapy. No wound improvement was observed.
Figure 1. Wound after 4 weeks of antimicrobial therapy. No wound improvement was observed.
Clinical reassessment of the wound identified potential chronic wound inflammation rather than infection, due to the lack of response to antimicrobial treatments. The patient’s care was transitioned to PROMOGRAN™ Matrix Wound Dressing, an ORC/collagen dressing. The patient showed significant improvement over the next 3 weeks and complete wound reepithelialization (Figure 2).
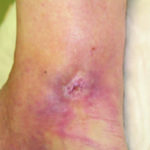 Figure 2. Wound full healed after 3 weeks of PROMOGRAN™ Matrix Wound Dressing use.
Figure 2. Wound full healed after 3 weeks of PROMOGRAN™ Matrix Wound Dressing use.
CONCLUSION
It can be challenging to distinguish between inflammation and infection in the chronic non-healing wound, as many of the clinical signs are similar between the two. The use of PROMOGRAN™ Matrix dressings in the presented case led to healing in a wound where treatments for infection were not having an impact.
References
1.Sorg H; Tiklorn DJ; Hager S; Hauser J; Mirastschijski U. Skin wound healing: an update on the current knowledge and concepts. Eur Surg Res. 2017;58:81-94.
2.World Union of Wound Healing Societies (WUWHS). Principles of best practice: Wound infection and clinical practice: an international consensus. London: MEP LTd, 2009. Available from www.mepltd.co.uk.
3.Swanson T; Angle D; Sussman G; et al. Wound infection in clinical practice. Wounds Int. 2016;1-29.
4.Passalacqua KD; Charbonneau M-E; O’Riordan MXD. Bacterial metabolism shapes the host: pathogen interface. Microbiol Spectr.2016;4: doi:10.1128/microbiolspec.VMBF-0027-2015.
5.Zhao G; Usui ML; Lippman SI; et al. Biofilms and inflammation in chronic wounds. Adv Wound Care. 2013;2:389-399.
6.Limoli DH; Jones CJ; Wozniak DJ. Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol Spectr.2015;3:MB-0011-2014. doi:10.1128/microbiolspec.MB-0011-2014.
7.Barker JC; Khansa I; Gordillo GM. A Formidable foe is sabotaging your results: what you should know about biofilms and wound healing. Plast Reconstr Surg. 2017;139:1184e-1194e.
8.Landen NX; Li D; Stahle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci.2016;73:3861-3885.
9.MacLeod AS; Mansbridge JN. The innate immune system in acute and chronic wounds. Adv Wound Care (New Rochelle). 2016;5:65-78.
10.Röhl J; Zaharia A; Rudolph M; Murray RZ. The role of inflammation in cutaneous repair. Wound Practice and Research. 2015;23:8-15.
11.Dunnill C; Patton T; Brennan J; et al. Reactive oxygen species (ROS) and wound healing: the functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int Wound J. 2017;14:89-96.
12.Zhao R; Liang H; Clarke E; Jackson C; Xue M. Inflammation in chronic wounds. Int J Mol Sci. 2016;17:2085. doi:1.3390/ijms17122085.
13. Wu. S; Applewhite AJ; Niezgoda J; et al. Oxidized regenerated cellulose/collagen dressings; review of evidence and recommendations. Adv Skin Wound Care. 2017;30(11S suppl1):S1-S8.
14. Schultz G; Bjarnsholt T; James GA; et al. Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair Regen. 2017;25:744-757.
15. Schafer M; Werner S. Oxidative stress in normal and impaired wound repair. Pharmacol Res 2008;58:165-171.
16. Rayment EA; Upton Z; Shooter GK. Increased matrix metalloproteinase-9 (MMP-9) activity observed in chronic wound fluid is related to the clinical severity of the ulcer. Br J Dermatol. 2008;158:951-961.
17.Cullen B; Watt PW; Lundqvist C; et al. The role of oxidised regenerated cellulose/collagen in chronic wound repair and its potential mechanism of action. Int J Biochem Cell Biol. 2002;34:1544-1556.

