
Lyndhia is a board certified Wound Ostomy (CWON) nurse at a major cancer center in New York City. She has twenty-five years of oncological experience in an acute-care setting. She works as a unit-based WOC nurse on the Colorectal and Gastric Mixed Tumor service for the past 12 years. Her area of expertise includes the management of complex surgical wounds, enterocutaneous fistulas, & ostomies. Lyndhia has designed & coordinated many educational programs for staff nurses, licensed independent practitioners (LIPs), and WOC nurses.
Isaac_Current Dialogues in Wound Management_2019_Special Spring Edition
NOTE: As with any case study, the results and outcomes should not be interpreted as a guarantee or warranty of similar results. Individual results may vary depending on the patient’s circumstances and condition.
INTRODUCTION
Over the past 8 years, the evolution of negative pressure wound therapy with instillation (NPWTi) has offered clinicians an effective tool to manage a plethora of complex wound types including pressure injuries, diabetic foot ulcers, and other chronic and acute wounds in different anatomical locations.1 NPWTi promotes granulation tissue formation by assisting with wound cleansing along with the removal of exudate, debris, and infectious materials. The adaptation of this therapy in current practice integrates negative pressure with automated, intermittent volume-controlled instillation of a topical wound cleansing solution with a dwell time for a prescribed period (NPWTi-d, V.A.C VERAFLO™ Therapy) followed by a user-defined length of negative pressure wound therapy (NPWT). This modality helps to cleanse wounds, loosen exudate, and remove these materials during the NPWT phase.2,3 According to Ahearn, intermittent pressure may lead to granulation tissue in lieu of continuous NPWT.4 In a porcine study, wounds treated with NPWTi-d with saline solution were reported to have 43% thicker granulation tissue compared with wounds treated with standard NPWT in continuous mode.5 However, these results have not been confirmed in human studies.
The aforementioned benefits of NPWTi-d come with a few challenges with maintaining a seal when patients are incontinent, wounds have increased drainage, and patients are diaphoretic. For many clinicians, these challenges can be laborious and time-consuming when attempting to establish a seal. This article will provide clinicians with some recommendations based on personal experience and will identify a few ancillary supplies to help overcome these hurdles using 2 case studies that help demonstrate the potential benefits of NPWTi-d.
CASE STUDY #1
Background
A 69-year-old obese female, who had undergone abdominal perineal resection with sacrectomy in October 2016, presented for care. Her post-operative course was complicated by a myocardial infarction and she was transferred to another hospital for cardiac catheterization. Once the patient returned to our facility for post-care, the perianal wound was found to be infected and required sharp debridement. This was performed at the bedside due to the patient’s poor cardiac status.
Description
The sacral wound measurement was 7 cm x 3 cm x 5 cm with undermining from 4-7 o’clock of 9 cm (see Figure 1). The wound base was intact and no tunnel was noted. The periwound bed was also intact, and the wound bed had 50% nonadherent slough/necrotic tissues and 50% granulation tissue with odor present. The coccyx bone was visible in the upper aspect of the wound. Drainage from the wound was pale brown in color. After consultation with cardiology, subsequent operating room debridement was warranted to remove any devitalized tissues. Our center initiated the use of V.A.C. VERAFLO™ Therapy in the operating room after the debridement.
 Figure 1. Wound before application of V.A.C. VERAFLO™ Therapy.
Figure 1. Wound before application of V.A.C. VERAFLO™ Therapy.
Tips for the Incontinent Patient
Of note, this patient suffered from stress urinary incontinence. This unintentional leakage of urine can interfere with the NPWTi-d dressing integrity. To mitigate any seal failure between the perineum region using a liquid skin protectant such as Marathon® (Medline Industries, Inc., Northfield, IL) can offer long-lasting protection. It is important to review the patient’s allergies to any adhesive to avoid any skin reactions. In the presence of skin damage, patients often complain of a burning sensation with direct contact from liquid skin protectant. Lightly apply Stomahesive® powder (ConvaTec, Inc, Greensboro, NC) over the skin irritation and follow with the application of a liquid skin sealant. To fill the gap in the perineum area and facilitate the absorption from surrounding drainage or urine, use an eakin Cohesive® Seal (ConvaTec) to improve the dressing wear time (see Figures 2 and 3). This cohesive ring is moldable and will fill any intricate contours or folds. In the event the tail-end of the dressing from the perineum becomes moist, apply a thin spread of paste over the marginally compromised area and let it dry before applying Tegaderm™ (3M) for reinforcement (see Figure 4).
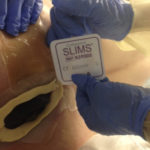 Figure 2. Application of ostomy paste and eakin Cohesive Slims® (ConvaTec).
Figure 2. Application of ostomy paste and eakin Cohesive Slims® (ConvaTec).
For patients with fecal incontinence, a bowel management device should be considered, if appropriate. This device recommendation will help reduce moisture-associated skin damage. In addition, lowering the amount of topical wound solution can facilitate durable dressing integrity. Meticulous skin care and patient assessment are necessary during instillation therapy. For the dressing application, simply follow the tips discussed earlier for urinary incontinence.
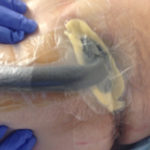 Figure 3. Application of barrier before bridging.
Figure 3. Application of barrier before bridging.
Dressing Selection
V.A.C. VERAFLO™ Therapy dressings are specifically designed for instillation therapy. These dressings utilize a new reticulated open-cell foam with a greater tensile strength and are more hydrophilic, which helps to provide better fluid distribution across the wound bed during the instillation phase. The options available include the: V.A.C. VERAFLO™ Dressing, V.A.C. VERAFLO Cleanse™ Dressing, and V.A.C. VERAFLO CLEANSE CHOICE™ Dressing. At our center, for wounds that require cleansing and granulation we consider the V.A.C. VERAFLO™ Dressings. For wounds with the requirements noted and that have thick exudate, slough, infectious materials, or when wound debridement is delayed, V.A.C. VERAFLO CLEANSE CHOICE™ Dressings have worked well. For wounds with complex geometries, tunnels, and undermining, V.A.C. VERAFLO CLEANSE™ Dressings are a good option.
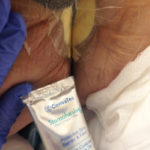 Figure 4. Application of a thin coat of skin protectant paste prior to placement of Tegaderm dressing.
Figure 4. Application of a thin coat of skin protectant paste prior to placement of Tegaderm dressing.
Dressing Case #1
Dressing to fill the tunnels and the wound base. We also bridged the V.A.C. Pad away from the wound by bridging the foam up over the patient’s hip. Practice Pearl: The patient did not like the feeling of the room temperature solution traveling across her hip and down the bridge. Given her dislike of the cool feeling during instillation cycles, the skin contact layer was changed from Tegaderm™ (3M, St. Paul, MN) to a thicker hydrocolloid skin contact for the bridge base. The thickness of the hydrocolloid base increased the patient’s comfort and allowed V.A.C. VERAFLO Therapy to continue. In our experience, this complaint is rare and Tegaderm™ (3M) or V.A.C.® Drape is usually sufficient (see Figures 5 and 6).
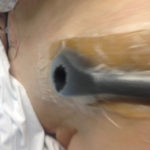 Figure 5. Hole cut in foam.
Figure 5. Hole cut in foam.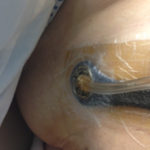 Figure 6. Placement of V.A.C. VERAT.R.A.C.™ Pad in the direction of the wound.
Figure 6. Placement of V.A.C. VERAT.R.A.C.™ Pad in the direction of the wound.
Topical Wound Solution Selection
A number of solutions are compatible with V.A.C. VERAFLO™ Therapy. In published guidelines, clinicians have used the following solutions: Prontosan® (surfactant and 1% betadine) (B. Braun Medical, Inc., Bethlehem, PA), Lavasept® (polyhexanide 0.04%) (B. Braun Medical, Inc.), Dakin’s (quarter strength 0.125 %) Nebacetin, Saline, and Bacitracine solution.8Nevertheless, Kim et al suggested that 0.9% normal saline may be just as effective as antiseptic Prontosan® (B. Braun Medical) for NPWTi when used in the management plan of infected wounds along with appropriate wound care, including debridement and systematic antibiotics.
Solution for Case #1
As a clinician, the topical wound solution choice is determined by each individual patient’s needs. In the presence of odor in the wound bed, a quarter strength 0.125% Dakin’s Solution® (Century Pharmaceuticals, Inc., Indianapolis, IN) was used for one week. Careful attention should be focused on the periwound bed to prevent skin damage given the nature of the topical wound solution. Protecting the periwound with a hydrocolloid barrier or eakin Cohesive Slims® (ConvaTec) will help prevent skin damage from the topical wound solution. After using Dakin’s Solution® (Century Pharmaceuticals) for 1 week, the NPWTi-d was continued with normal saline for an additional 2 weeks. After 3 weeks of treatment, the size of the wound decreased and the patient was clinically ready for discharge.
Setting Volume, Dwell time, Pressure Phase Selection
In this phase, patients should be made aware that as the topical wound solution begins to fill the wound, it will feel cold but not wet. Using the Fill Assist mode to determine the volume will depend on the wound size. Carefully observe the foam – it should become darker in color and be no more than 80% saturated. Too much fluid will cause leakage. We find that pre-moistening the foam and squeezing out any excess solution can be helpful. If leakage occurs at the chosen volume, lower the amount by 20%. Once the volume is set, the V.A.C. VERAFLO™ Therapy automatically proceeds to the instillation phase. During the course of this patient’s treatment, the dwell time (soak phase) was set for 5 minutes while using quarter strength 0.125% Dakin’s Solution® (Century Pharmaceuticals) and was extended to 10 minutes while using saline solution. A shorter dwell time could be used for significant urinary incontinence. There is very little scientific evidence for optimal dwell time and it is dependent on individual cases. The negative pressure phase or the length of time that negative pressure is applied is at the clinician’s discretion. In this case, negative pressure phase was 5 hours in part because of the type of topical wound solution used, the presence of urinary incontinence, and the wound location in the sacral area. The pressure setting for negative pressure remained at the default setting of -125 mmHg.
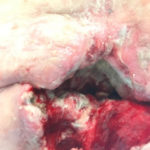 Figure 7a. Wound at presentation.
Figure 7a. Wound at presentation.
Outcome
The goal for this wound was to improve granulation tissue. The patient did not need further wound debridement. She was transitioned home with NPWT for 6 weeks and complete closure was achieved. Key points to Share with Patient and Staff
•Inform patients to call nursing staff for leakage and alarm situations.
•For leakage, staff can suggest the license independent practitioner (LIP) decrease the instill volume by 20% and reinforce the dressing as needed.
•If the source of the leakage cannot be resolved, then staff can suggest the LIP temporarily switch therapy to NPWT if managing service is not available.
•Check the alarm and therapy alarm history to retrieve any information.
•Have extra canisters available at the bedside.
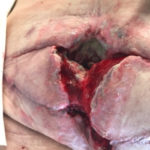 Figure 7b. Wound after 1 week of V.A.C. VERAFLO™ Therapy and surgical debridement.
Figure 7b. Wound after 1 week of V.A.C. VERAFLO™ Therapy and surgical debridement.
CASE STUDY #2
Background
This is a 59-year-old obese male with rectal cancer who had undergone lower anterior resection with chronic leak and other failed interventions. The patient later required an abdominal perineal resection with gluteal flap in 2017 which eventually failed, and the superior wound tracked to the pelvis and prostate. His past medical history included diabetes and hypertension. The inferior part of the wound was superficial. With the presence of exposed organs, the wound was managed with V.A.C. VERAFLO™ Therapy and two ADAPTIC TOUCH™ Non-Adhering Silicone Dressings were at the wound base. He was on a specialty bed because of the flap and was not compliant with his ambulation.
Description
There was no major improvement with the wound. Three weeks later the patient reported increased pain in the coccyx bone. The wound measured 18 cm x 11 cm x 13 cm at the time (see Figure 7). At this stage, orthopedics was consulted. A subsequent MRI was negative. A 6-week course of intravenous antibiotics was initiated for possible osteomyelitis. The plastic surgery team was highly suspicious of the likelihood of a pressure injury in the coccyx region since the remaining gluteal flaps healed well. The wound base was covered with granulation tissue (see Figure 8). The V.A.C. VERAFLO™ Therapy was started when the wound size measured 17 cm x 10 cm x 11 cm. Since the patient was in bed most of the day, there was a lot of moisture present after the first dressing removal.
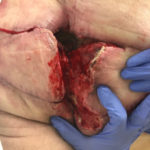 Figure 8. Wound after 2 weeks of V.A.C. VERAFLO™ Therapy
Figure 8. Wound after 2 weeks of V.A.C. VERAFLO™ Therapy
Tips for Patients with Diaphoresis
For excessive moisture, 3M™ Cavillon™ Advanced Skin Protectant (3M) offers a superior skin barrier for moderate-to-severe skin damage and this was applied prior to the dressing application. eakin Cohesive Slims™ (ConvaTec) were also used to fill any intricate areas such as the gluteal cleft and perineum. Window framing the periwound was essential to create a durable seal. Applying an ostomy paste at the distal portion of the perineum and letting the alcohol dissipate are recommended before covering it with another moisture-resistant drape or Tegaderm™ (3M) that has been cut in small pieces. In this case, the skin protectant helped to maximize the life of the dressing
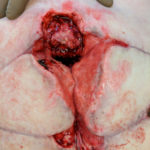 Figure 9. Wound after bilateral gluteus maximus myocutaneous advancement flap.
Figure 9. Wound after bilateral gluteus maximus myocutaneous advancement flap.
Dressing Selection for Case #2
To fill this deep irregular contour, the wound was loosely packed with V.A.C. VERAFLO CLEANSE™ Dressing. V.A.C. VERAFLO™ Dressing was used for bridging the V.A.C. VERAT.R.A.C™ Pad onto a non-pressure surface. For a larger wound it is best to use the V.A.C. VERAT.R.A.C Duo™ Tube Set.
Solution, Setting Volume, Dwell time, Pressure Phase Selection
The initial goal was to clean the wound. Our NPWTi-d approach consisted of instillation of quarter strength 0.125% of Dakin’s Solution® (Century Pharmaceuticals (120 mL), with 5 minutes of dwell time, followed by 4 hours of continuous NPWT at -125 mmHg. One week after V.A.C. VERAFLO™ Therapy was initiated, the patient went to the operating room for a wound debridement where part of the coccyx bone was excised. The wound’s final defect measured 15 cm x 8 cm x 9 cm; extending now 9 cm cephalad from the coccyx (versus 11 cm at the last NPWTi-d). The V.A.C. VERAFLO™ Therapy was continued using normal saline (100 mL), with 5 minutes of dwell time, followed by 4 hours of NPWT. After 2 more weeks, the patient went to the operating room for a debridement and bilateral gluteus maximus myocutaneous advancement flap (see Figures 8 and 9). The cavity could have handled more fluid, but the decision was dependent on the excess moisture and maceration.
Outcome
This case was unique and challenging because of the patient’s poor compliance with ambulation, which may have contributed to delayed wound healing and the development of a pressure injury. The patient was discharged to a rehabilitation facility and the sacral flap was intact. The flap remained intact a year later.
Conclusion
Managing wounds requires a comprehensive assessment by addressing physiological factors as well as intrinsic and extrinsic factors. Failure to address these factors can have a negative outcome on wound healing. In our center’s experience, NPWTi-d is beneficial for the management of various wounds. The solution choice and settings are tailored to each patient. For the patient who is incontinent or diaphoretic, the adaptation of accessory products can help ensure the full function and benefit of the therapy. This treatment modality was proven efficacious for the 2 complex case studies presented here. In these instances, wounds went to complete wound closure and did not require a readmission or additional debridement.
References
1.Argenta LC, Morykwas MJ. Vacuum-assisted closure: A new method for wound control and treatment: Clinical experience. Ann Plast Surg.1997;38(6):563-576.
2. Wolvos T. The use of negative pressure wound therapy with an automated, volumetric fluid administration: An advancement in wound care. Wounds. 2013;25(3): 75-83.
3. Gabriel A. Integrated negative pressure wound therapy system with volumetric automated fluid instillation in wounds at risk for compromised healing. Int Wound J. 2012;9 Suppl 1:25-31. doi: 10.1111/j.1742-481X.2012.01014.x.
4. Ahearn C. Intermittent NPWT and lower negative pressures exploring the disparity between science and current practice: A review. Ostomy Wound Manage. 2009;55(6):22-28.
5. Lessing MC, James RB, Ingram SC. Comparison of the effects of different negative pressure wound therapy modes – continuous, noncontinuous, and with instillation – on porcine excisional wounds. Eplasty. 2013;13:e51.
6. Téot L, Boissiere F, Fluieraru S. Novel foam dressing using negative pressure wound therapy with instillation to remove thick exudate. Int Wound J. 2017;14(5):842-848. doi: 10.1111/iwj.12719.
7. Kim PJ, Applewhite A, Dardano AN, et al. Use of a novel foam dressing with negative pressure wound therapy and instillation: Recommendations and clinical experience. Wounds. 2018;30(3 Suppl):S1-S17.
8. Kim PJ, Attinger CE, Steinberg JS, et al. Negative-pressure wound therapy with instillation: International consensus guidelines. Plast Reconstr Surg. 2013;132(6):1569-1579. doi: 10.1097/PRS.0b013e3182a80586.
9. Kim PJ, Attinger CE, Oliver N, et al. Comparison of outcomes for normal saline and an antiseptic solution for negative-pressure wound therapy with instillation. Plast Reconstr Surg. 2015;136(5):657e-664e. doi: 10.1097/PRS.0000000000001709.
10. Mckanna M, Geraci J, Hall K, et al.Clinician panel recommendations for use of negative pressure wound therapy with instillation. Ostomy Wound Manage. 2016;62(4):S1-S14.

