
Dr. Robert Snyder is Professor and Director of Clinical Research and Fellowship Director in wound care and research at Barry University SPM. He has created and implemented the first post-residency Wound Management and Research Fellowship for podiatric medical schools. He is certified in foot and ankle surgery by the American Board of Podiatric Surgery and is also a board-certified wound specialist. Dr. Snyder is past-president of the Association for the Advancement of Wound Care and past-president of the American Board of Wound Management, the certifying body for Wound Care Specialists. In addition to his doctorate, he holds an MSc in Wound Healing and Tissue Science from Cardiff University. His expertise at Cardiff, Wales, was further acknowledged as he was selected as an Internal Marker for MSc dissertations with an Honorary Title. To constantly expand his knowledge and stay current in all aspects of healthcare, he is completing an MBA in Health Management. Dr. Snyder is a key opinion leader and sought-after speaker, lecturing extensively throughout the United States and abroad. He was chosen to develop and teach a wound care course for physicians internationally. Dr. Snyder has published several book chapters and over 150 papers in peer-reviewed and trade journals on wound care. He serves on the editorial advisory boards of Ostomy Wound Management, Wounds and Podiatry Management and has recently been appointed as a periodic reviewer for the Lancet and NEJM. He has been a Principal Investigator on more than 50 randomized controlled trials for innovative wound healing modalities and products.

Dr. Ead is currently a Resident Physician (PGY-1) at Westside Regional Medical Center his training incorporates all aspects of podiatric medicine & surgery with an emphasis on joint replacement, external fixation, arthroscopy, trauma, tibial shaft/plafond, ankle, hindfoot and forefoot reconstruction. Prior to residency, he received his Doctorate in Podiatric Medicine at Barry University School of Podiatric Medicine and his master’s degree in biomedical science with a concentration in Wound Healing and Tissue Regeneration. Dr. Ead has been involved in various research studies and has worked closely with wound care specialist Dr. Robert Snyder DPM, MSc, CWS.

Dr. Cherison Cuffy is a graduate of Temple University School of Podiatric Medicine. He then completed a three-year podiatric medicine and surgery residency at Northwest Medical Center in Margate Florida. Dr. Cuffy is currently a faculty member at Barry University School of Podiatry as an assistant professor. He now participates in the clinical education of the podiatry students along with a focus on clinical research at the Paul and Margaret Brand Research Center.
Snyder_Current Dialogues in Wound Management_2020_Article_4
OVERVIEW
The prevalence and costs of lower extremity ulcerations in the US are increasing. Venous leg ulcerations (VLU) are the most common type of leg ulcer, affecting around 1% of the population and 3% of people aged over 80.1 Ischemic ulcerations, also referred to as arterial ulcerations, are primarily attributed to poor circulation/perfusion to the lower extremities. The overlying soft tissues are deprived of oxygen desiccating the area of vital nutrients and healing capabilities that subsequently form an open wound.
There are a number of causes of lower extremity ulcerations. Additionally, there is even a greater problem in patients who may have several causes for lower extremity ulcerations that evolve with time. For instance, a patient with a long-standing, pure venous ulcer may have arterial insufficiency develop with increasing age, which can make the treatment pathway challenging and problematic if not correctly identified.
VENOUS LEG ULCERATIONS
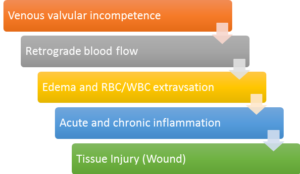
The Society of Vascular Surgery and American Venous Forum proposed the standard definition of the VLU as an open skin lesion of the leg or foot that occurs in an area affected by venous hypertension.2 Diagnosis of venous leg ulcerations is made by clinical history and examination, and anatomic and physiologic data to confirm venous etiology and rule out other causes, most notably arterial insufficiency. The etiology is attributed to a series of local and systemic factors that include venous valvular incompetence, retrograde blood flow, and venous hypertension (Figure 1). Clinically this can be appreciated with increased lower extremity edema and swelling. These factors lead to an increase in pressure within the deep venous system causing the vein walls to stretch, opening up the valves, and allowing even more blood to fill the veins.1
Another factor that influences the development of VLUs is calf muscle pump failure. The calf muscle, through contraction and relaxation, aids in the flow of blood back to the heart through the veins. As the ankle is plantar flexed, the shape of the calf muscle changes, becoming wider and flat, exerting pressure on the veins.1 Failure of this mechanism causes venous stasis of blood and the aforementioned increased venous pressure.1 Calf pump failure can potentially arise from paralysis, immobility, sleeping in a chair with legs dependent for long periods of time and fixed ankle joints.1
Other potential VLU etiologies include the fibrin cuff and blood cell activation and trap hypothesis theories. The fibrin cuff theory stipulates that capillary bed distension permits fibrinogen to leak into the dermal tissue forming fibrin cuffs around dermal capillaries and reducing fibrinolytic activity obstructing the healing process.3 The white blood cell activation theory hypothesizes that venous hypertension reduces velocity of blood through capillary beds resulting in leukocytes adhere to each other and/or capillary walls.4 Leukocyte aggregation causes “plugging” of the capillaries, resulting in tissue ischemia/ migration of cells into the surrounding tissues where they release proteolytic enzymes/inflammatory mediators causing additional tissue damage.4 Finally, the trap hypothesis states that fibrin and macro-molecule network leak out of the permeable capillary beds into the dermis traps growth factors and matrix proteins, rendering them unavailable. Many healthcare providers believe that it is actually combination of these potential mechanisms that lead to the recalcitrant nature of these chronic wounds.
Venous Leg Ulcer Diagnosis
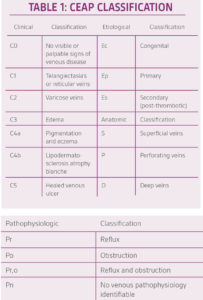
The diagnosis of VLUs is made clinically on the basis of anatomic location, morphology, and characteristic skin changes. Once veins become distended, the venous wall starts to disintegrate often resulting in edema, hemosiderin formation, hyperpigmentation, lipodermatosclerosis, and prominent venous ulcers. Patients with venous insufficiency commonly present an inverted champagne bottle look on the effected leg. It should be noted that the appearance of venous insufficiency could vary. Distention of the veins in the sub-dermal plexus results in varicosities. When smaller sub-dermal capillary networks distend, they form telangiectasia’s also known as “spider veins”. Cellular senescence has been reported in fibroblasts collected from chronic non-healing ulcers. Lal et al. found that fibroblasts from patients with increasing levels of venous disease by clinical class, etiologic, anatomic, and pathophysiologic (CEAP) criteria displayed a progressively diminishing response to agonist-induced proliferation.5
In order to standardize the reporting and treatment of the diverse manifestations of chronic venous disorders, a comprehensive classification system (CEAP) has been developed to allow uniform diagnosis and comparison of patient populations (Table 1).2 The fundamentals of the CEAP classification include a description of the clinical class (C) based upon objective signs, the etiology (E), the anatomical (A) distribution of reflux and obstruction in the superficial, deep and perforating veins, and the underlying pathophysiology (P), whether due to reflux or obstruction.
Other conditions may coexist in a patient with chronic venous insufficiency. A key differential is a Marjolin’s ulcer, which may develop when squamous transformation occurs in a preexisting benign chronic wound. A VLU not responding to standard wound care and compression after 4-6 weeks of therapy should be biopsied. The Marjolin’s ulcer, other skin neoplasms, vasculitis, vasculopathy, and inflammatory ulcers are differentials to name a few which can be the primary etiology of the ulcer or coexist in a patient with venous hypertension.
Venous Leg Ulcer Management
The mainstay treatment for VLUs is compression therapy. Gross arterial disease should be ruled out. The degree of compression must be modified when mixed venous/arterial disease is confirmed during the diagnostic work-up. It is important to understand how the diagnosis was made and to understand the limitations of the method. Color duplex ultrasound scanning can be performed to confirm a venous etiology. Venous plethysmography, computed tomography venography, magnetic resonance venography, contrast venography, and intravascular ultrasound may be necessary if duplex venous ultrasound is nondiagnostic. The additional venous imaging can assist in the assessment of venous hypertension due to venous outflow obstruction caused by thrombotic or non-thrombotic iliac vein obstruction.2 Providers may also opt to use a class high-compression system (three layers, four layers, ‘short stretch’) or paste-containing bandages– modified when venous/arterial disease is confirmed.6 Finally, intermittent pneumatic pressure (IPC) can be used with or without compression dressings.6
Adhering to evidence-based wound bed preparation practices is vital for optimal healing. Debride all necrotic or devitalized tissue, control infection (if present), maintain a healthy moisture balance, and maintain proper wound edge and depth. The rate of wound healing should be evaluated to determine whether treatment is optimal and whether progress is being made. Use a dressing that will maintain a moist wound-healing environment, manage exudate, protect peri-ulcer skin, and be cost effective.7 The dressing material or platform is also important to ensure sustainability and efficacy of the antimicrobial effect. For example, even in low concentrations, ionic silver (Ag+) is highly efficacious on microorganisms in vitro.7
The use of cellular therapies, tissue matrices, human matrices, and skin-substitutes should be considered when the VLU has failed to show signs of healing.7 To decrease the recurrence of venous ulcers, radiofrequency or laser ablation of the incompetent superficial veins in addition to compression therapy is strongly recommended.2 Vascular interventionists can offer various options with the use of the CEAP classification and venous ulcer risk including ultrasound-guided sclerotherapy, endo-venous ablation, vein ligation, vein transposition or transplantation, primary valve repair, or open surgery.2 Adequate nutrition is important to support wound healing including vitamins, minerals and protein.
Clinical Case
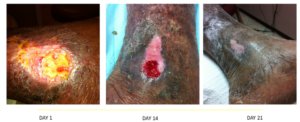
A 63-year-old male presented to the university research clinic with a recurrent venous leg wound overlying the gaiter region of the left medial malleolus. The patient had a past medical history significant for chronic venous insufficiency with lower extremity edema, cardiovascular disease, asthma, and obesity. The patient remarked that he discontinued wearing his compression stockings and subsequently developed another venous ulceration that formed approximately 1-2 months ago. After the patient-centered concerns and underlying pathologies had been addressed, and the wound bed had been adequately prepared, a treatment plan was developed to address the systemic and local factors instigating the chronicity of this pathology (Figure 2). Initial treatment modalities included compression therapy, non-adherent antimicrobial alginate dressings for two weeks followed by robust collagen-based dressing use until wound closure was observed. Adjunctive therapies including the use of pentoxifyllene and micronized purified flavonoid fraction along with compression were utilized. The combination of these treatment modalities has been reported to potentially help increase the healing rate of chronic venous ulcers.8 Although the physiology and mechanism of action is not completely understood, these two drugs interact synergistically on leukocyte activation to protect the microcirculation from the deleterious effect of venous hypertension.2 The patient healed eventfully after 21 days of treatment.
Arterial Lower Extremity Ulcers
Arterial leg ulcers occur as a result of reduced arterial blood flow and subsequent tissue perfusion. Atherosclerosis or peripheral vascular disease is the most common cause of arterial leg ulceration.9 Atheroma or plaque development can be caused by tobacco use, obesity, hyperlipidemia, hypertension and/or diabetes. Arterial leg ulcers usually affect men over 45 years and women over 55 years.10 As the arterial blood supply reduces, patients experience an increase in pain. Their ability to walk distances and uphill causes pain, which is also present on leg elevation. This is known as intermittent claudication. The treatment of patients with chronic non-healing leg ulcers associated with arterial insufficiency involves a critical assessment of the patient’s suitability for invasive procedures and the potential risk of limb loss based on ulcer characteristics.
Arterial ulcerations occur distal to impaired arterial supply most commonly on the lateral aspect of the leg, when presenting on the lower extremity. However, they can occur anywhere that is susceptible to trauma such as the interphalangeal joints of the toes. Wound margins are even, sharply demarcated and punched out and have minimum exudate and are very painful in nature.11 The Society for Vascular Surgery Lower Extremity Guidelines Committee formed a classification system to take into account the change in demographics over the years, the increased incidence of diabetes mellitus in this population, and the advances in revascularization techniques.12
Specifically, this protocol was developed to support clinicians in their management of patients whose comorbidities increase their risk of lower extremity amputation.12 The three main risk stratification factors for arterial ulcers are: wound extent, ischemia, and foot infection (WIfi). Hicks et al found that with the progression through each stage of WIfi there is a strong association with prolonged wound healing time, a higher number of surgical procedures, and an increased cost of care.13
Treatment of Patients with Mixed Arterial and Venous Ulcers
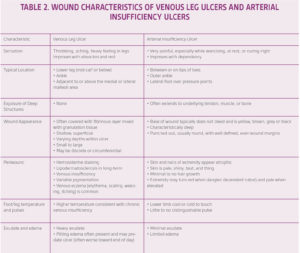
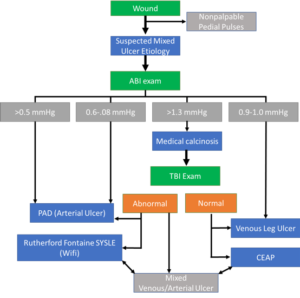
A thorough clinical history, focusing on the duration and size of the ulcer with any associated lower extremity symptoms is the first step in patient management. In clinical studies, the reported incidence of arterial insufficiency in patients with VLUs has ranged from 15% to 30%.10 Utilizing an evidence-based diagnostic guideline is critical in order to differentiate between the two etiologies (Figure 3). A comprehensive evaluation of the patient’s comorbidities and overall health is vital, as patients with arterial disease are likely to be older with significant cardiovascular comorbidities. Typical characteristics of each type of leg ulcer are described in Table 2 to help guide accurate diagnosis.11 The literature has been clear in regard to reducing the strength of compression for treatment of VLUs. However, it is critical in patients with significant arterial insufficiency to minimize the risk of compression-related complications including pain and compression-related necrosis. The exact level of arterial insufficiency that warrants modified compression is debatable, ranging from 0.7 mmHg to 0.9 mmHg.10 Tissue necrosis typically occurs over bony prominences including the malleoli and anterior tibia, or over the posterior heel and tendons.11 Additional padding is recommended to these areas underneath compression to reduce the potential risk of tissue injury. Most experts recommend against high strength compression in patients with an ankle pressure below 80 mmHg or ABI below 0.7 mmHg to 0.85 mmHg.10 If the ankle pressure is below 60 mmHg or the ABI is below 0.5 mmHg, revascularization is recommended prior to compression therapy.10 Furthermore, doppler ultrasound to measure ankle-brachial pressure index should be performed when the ulcer is regressing, no signs of healing by 12 weeks, sudden increase in size of ulcer, sudden increase in pain, foot color or temperature change, or there is recurrence of ulcer. 6, 14
CONCLUSION
A comprehensive methodology of wound assessment, treatment of patient- and wound-centered concerns, and follow-up should be adhered to for all chronic wounds, irrespective of wound type. Patient-centered factors including nutrition, etiology, obesity management, nicotine use, uncontrolled infection, circulation, incontinence, pain, and psychosocial factors, among others, can all impede healing and need to be addressed for successful treatment. The management of patients with mixed ulcerative pathologies requires a multidisciplinary team approach, educating patients on issues of correct foot care, and the importance of seeking early medical advice. It cannot be overstated that venous disease and arterial disease can exist in the same patient, therefore utilizing evidence-based guidelines can only optimize patient outcomes.
Patient data and photos courtesy of Snyder, Ead and Cuffy.
As with any case study, the results and outcomes should not be interpreted as a guarantee or warranty of similar results. Individual results may vary depending on the patient’s circumstances and condition.
References
- Anderson I. Wound Essentials 1: Aetiology, assessment & management of leg ulcers. Wound Essentials 2010;1:20-24, 26, 28, 30, 32, 34, 36-37.
- O’Donnell TF, Jr., Passman MA, Marston WA et al. Management of venous leg ulcers: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg 2014;60(2 Suppl):3S-59S. doi:10.1016/j.jvs.2014.04.049.
- Burnand KG, Whimster I, Naidoo A, Browse NL. Pericapillary fibrin in the ulcer-bearing skin of the leg: the cause of lipodermatosclerosis and venous ulceration. Br Med J 1982;285(6348):1071-1072. doi:10.1136/bmj.285.6348.1071.
- Saharay M, Shields DA, Porter JB, Scurr JH, Coleridge Smith PD. Leukocyte activity in the microcirculation of the leg in patients with chronic venous disease. J Vasc Surg 1997;26(2):265-273. doi:10.1016/s0741-5214(97)70188-5.
- Lal BK, Saito S, Pappas PJ et al. Altered proliferative responses of dermal fibroblasts to TGF-beta1 may contribute to chronic venous stasis ulcer. J Vasc Surg 2003;37(6):1285-1293. doi:10.1016/s0741-5214(02)75295-6.
- O’Meara S, Tierney J, Cullum N et al. Four layer bandage compared with short stretch bandage for venous leg ulcers: systematic review and meta-analysis of randomised controlled trials with data from individual patients. Br Med J 2009;338:b1344. doi:10.1136/bmj.b1344.
- Snyder RJ, Fife C, Moore Z. Components and Quality Measures of DIME (Devitalized Tissue, Infection/Inflammation, Moisture Balance, and Edge Preparation) in Wound Care. Adv Skin Wound Care 2016;29(5):205-215. doi:10.1097/01.ASW.0000482354.01988.b4.
- Parsa H, Zangivand AA, Hajimaghsoudi L. The effect of pentoxifylline on chronic venous ulcers. Wounds 2012;24(7):190-194.
- Moffatt C. Leg Ulcers. In: Murray S, ed. Vascular Disease: Nursing and Management. 1st ed. John Wiley & Sons, Inc.; 2001:200-237.
- Grey JE, Harding KG, Enoch S. Venous and arterial leg ulcers. Br Med J 2006;332(7537):347-350. doi:10.1136/bmj.332.7537.347.
- Gupta S, Andersen C, Black J et al. Management of Chronic Wounds: Diagnosis, Preparation, Treatment, and Follow-up. Wounds 2017;29(9):S19-S36.
- Mills JL, Sr., Conte MS, Armstrong DG et al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg 2014;59(1):220-234. doi:10.1016/j.jvs.2013.08.003.
- Hicks CW, Canner JK, Mathioudakis N et al. The Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification independently predicts wound healing in diabetic foot ulcers. J Vasc Surg 2018;68(4):1096-1103. doi:10.1016/j.jvs.2017.12.079.
- Dogra S, Sarangal R. Summary of recommendations for leg ulcers. Indian Dermatology Online Journal 2014;5(3):400-407. doi:10.4103/2229-5178.137829.
- Snyder RJ, Ead JK, Cuffy C. Wound Assessment. In: Shah JB, Sheffield PJ, Fife CE, eds. Textbook of Chronic Wound Care: An Evidence-Based Approach to Diagnosis and Treatment. 1st ed. North Palm Beach, FL: Best Publishing Company; 2018:103-126.
@Copyright 2020 3M. All rights reserved. 3M and the other marks shown are marks and/or registered marks. Unauthorized use prohibited. PRA-PM-US-02373 (04/20).

