
Dr. Robert Snyder is Professor and Director of Clinical Research and Fellowship Director in wound care and research at Barry University SPM. He has created and implemented the first post-residency Wound Management and Research Fellowship for podiatric medical schools. He is certified in foot and ankle surgery by the American Board of Podiatric Surgery and is also a board-certified wound specialist. Dr. Snyder is past-president of the Association for the Advancement of Wound Care and past-president of the American Board of Wound Management, the certifying body for Wound Care Specialists. In addition to his doctorate, he holds an MSc in Wound Healing and Tissue Science from Cardiff University. His expertise at Cardiff, Wales, was further acknowledged as he was selected as an Internal Marker for MSc dissertations with an Honorary Title. To constantly expand his knowledge and stay current in all aspects of healthcare, he is completing an MBA in Health Management. Dr. Snyder is a key opinion leader and sought-after speaker, lecturing extensively throughout the United States and abroad. He was chosen to develop and teach a wound care course for physicians internationally. Dr. Snyder has published several book chapters and over 150 papers in peer-reviewed and trade journals on wound care. He serves on the editorial advisory boards of Ostomy Wound Management, Wounds and Podiatry Management and has recently been appointed as a periodic reviewer for the Lancet and NEJM. He has been a Principal Investigator on more than 50 randomized controlled trials for innovative wound healing modalities and products. Dr. Snyder is a consultant for 3M.

Dr. Ead is currently a Resident Physician (PGY-1) at Westside Regional Medical Center his training incorporates all aspects of podiatric medicine & surgery with an emphasis on joint replacement, external fixation, arthroscopy, trauma, tibial shaft/plafond, ankle, hindfoot and forefoot reconstruction. Prior to residency, he received his Doctorate in Podiatric Medicine at Barry University School of Podiatric Medicine and his master’s degree in biomedical science with a concentration in Wound Healing and Tissue Regeneration. Dr. Ead has been involved in various research studies and has worked closely with wound care specialist Dr. Robert Snyder DPM, MSc, CWS.
Synder_Current Dialogues in Wound Management_2020_Article_18
Introduction
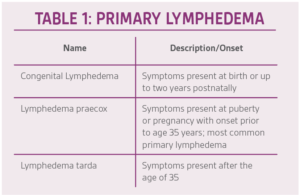
The lymphatic system shares some similar characteristics to its arterial and venous network counterparts. Lymphatic flow is able to circulate throughout the body via four distinct pathways: capillary blood pressure, osmotic, oncotic, and interstitial (hydrostatic) fluid pressures.1 The peripheral muscular compartments in conjunction with the venous system aid in peripheral retrograde flow. Lymphedema occurs when there is an accumulation of interstitial and fibro-adipose tissues causing peripheral swelling and edema. The etiology of lymphedema can be broken down into two separate categories; (primary) congenital malformation of the lymphatic system (Table 1) or (secondary) as a consequence of a medical condition and/or treatment of another unrelated comorbidity.2
Persistent accumulation of lymphatic fluid promotes proliferation of adipocytes and deposition of collagen fibers in the extracellular matrix and around capillary and collecting lymphatics.2 This can lead to a reduction in the numbers of lymph channels or obstruction of the available channels. Potential etiologies of secondary lymphedema include; chronic venous insufficiency, trauma/burns, infection, inflammatory disorders, cancer and cancer treatment among others.
Epidemiology and Risk Factors
Globally, the most common cause of lymphedema is filariasis.3 This pathology is caused by an infection of the source by a vector within the nematode species Wuchereria bancrofti.3 The Centers for Disease Control and Prevention estimate that lymphatic filariasis affects over 120 million people in 72 countries throughout the tropics and subtropics of Africa, the Western Pacific, Asia, and parts of the Caribbean and South America.3 In the developed world, the majority of cases of lymphedema are secondary and due to malignancy or its treatment (pharmacologic, surgical). Risk factors for lymphedema include certain hereditary syndromes, malignancies and their treatments, genetic mutations, obesity, autoimmune diseases, older age, and inflammatory arthritis.4 Additionally, older age and obesity contribute to an increased likelihood of worsening symptoms in patients already affected with lymphedema.
Cancer-related lymphedema is caused by an increased interstitial pressure through increased pressure against the lymphatic channels resulting in lymphedema,5 a phenomenon known as lymphangitic carcinomatosis. Additionally, any physical disruption or removal of lymph nodes at the time of surgery can increase the risk of lymphedema in patients with cancer. Several pharmacologic medications have a side effect profile that could also cause lymphedema. Breast cancer is the most common cancer associated with upper extremity lymphedema.4 The incidence of cancer-related lower extremity lymphedema varies by type of malignancy and treatment. In a systematic review that included 43 studies (genitourinary, gynecologic, lower extremity melanoma), the overall incidence of lower extremity lymphedema was estimated to be 20 percent.4
Lower Extremity Lymphatic Anatomy
Drainage into the lymphatic system starts in the lymphatic pre-collectors, which contain single-layer endothelial cells with anchoring filaments and valves.6 The stretching of these anchoring filaments opens clefts between cells to expand, allowing entry of interstitial fluid and macromolecules.6 From the lymphatic pre-collectors and collectors, lymph flows through lymphatic capillaries and, thereafter, flows into the lymph nodes, which concentrate and filter the lymph.6 Eventually the lymph returns to the cardiovascular system via the thoracic duct.6 It is the job of the lymphatic system to capture and drain this fluid, filter and concentrate it in the lymph nodes, and then return it to the vascular system.6 The lymph nodes of the lower extremities consist of the popliteal and inguinal nodes.
Lymphedema of the lower extremities can be primary or secondary, but secondary lymphedema is much more common in the lower extremity than primary lymphedema and is often associated with treatment of malignancy, trauma, or infection. Interestingly, isolated foot involvement is more likely to be seen with primary lymphedema. Lymphedema shares many clinical features with chronic venous insufficiency (CVI) and it is critical for the caregiver to distinguish if the edematous process is the result of a venous inefficiency, lymphatic insufficiency, or a combination of both. Lymphedema caused by insufficiency of both the venous and lymphatic systems is known as phlebolymphedema.6 CVI results in an excessive fluid load at the tissue level, creating additional load to the lymphatic system.6 The increase in lymphatic flow may become much greater than the lymph transport capacity. Additionally, lymphedema should not be confused with lipedema. Lipedema is a fatty tissue disorder, caused by an abnormal deposition of adipose tissue.
Clinical Evaluation
A comprehensive review of the patient’s medical history and holistic physical examination cannot be overestimated in the management of lymphedema. The history should include age of lymphedema onset, affected areas, details of disease progression, history of trauma, medical history, surgical history, and family medical history. Physical examination should assess swelling, skin changes, and infection (Figure 1).
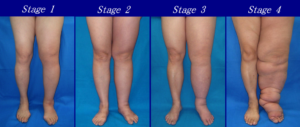
The physical examination includes an assessment of the skin, soft tissues, and vascular system of the affected limb(s) (Figure 2).
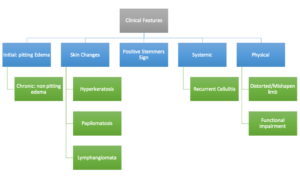
The inability or failure to pinch or pick up a fold of skin at the base of the toe (i.e. Stemmer sign) (Figure 3) is indicative of lymphedema complicated by skin fibrosis; however, a negative Stemmer sign does not rule out lymphedema.7
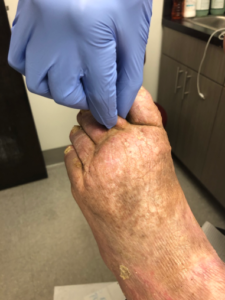
The patient’s overall functional status should be evaluated and assessed during the examination process. Some functional observations for patients with lower extremity lymphedema may include the presence of a limp, the use of an ambulation aid, the (in)ability to bend over to remove shoes/socks, and the (in)ability to make a forward stride. If functional limitations are noted, the patient should be referred to physical therapy. It is vital for clinicians to obtain lower extremity measurements by measuring four points in both the affected and contralateral extremities with the limb in a relaxed position. Key anatomical landmarks include metatarsal-phalangeal joints (if edematous), 2 cm superior to the medial malleolus, 10 cm above the superior pole of the patella, and 10 cm below the inferior pole of the patella.8
Treatment
Treatment for lymphedema is based on rerouting the lymph fluid through remaining functional lymph vessels. This is accomplished through elevation, exercises, compression garments and devices, and manual lymph drainage managed with absorbent dressings. Treatment combines the management of drainage with good skin care practices.
The initial approach for the management of extremity lymphedema (upper or lower) begins with conservative management, which involves a combination of self-care (i.e. skin care, weight management), physiotherapy, and compression therapy (i.e. compression bandaging, compression garments, ulcer dressings).9-11 While these treatments do not address the underlying cause of lymphedema, they can control swelling and prevent development of long-term sequelae such as irreversible skin changes (i.e. elephantiasis).9-11 Many patients with mild-to-moderate lower extremity lymphedema may be adequately controlled with conservative measures. However, in some cases, surgical intervention such as liposuction, lympho-venous bypass, and vascularized lymph node transfer surgery may be required and can be effective in relieving symptoms while improving quality of life.12 Skin grafting may also be indicated in recalcitrant cases to close open ulcerations after wound bed preparation has been accomplished.13
Case Report
A 68-year-old male presented with lymphedema and multiple, copiously draining ulcerations in the right lower extremity (Figure 4).
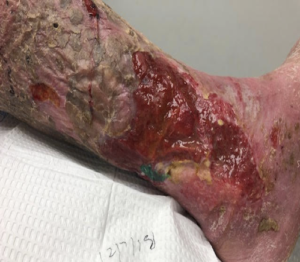
Symptoms were present for years and failed to respond to compression, foam dressings, and abdominal pads (Figure 5).
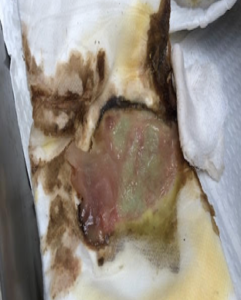
The drainage became so profuse that the patient would ‘puddle’ fluid on the floor, soaking the dressing and precluding sustained compression. A positive Stemmer sign was noted, and the patient had palpable pedal pulses with mild symptoms of venous insufficiency. The patient’s orthopedic and neurological status was unremarkable. The patient suffered from hypertension requiring use of anti-hypertensive medication; however, his medical history was otherwise absent of underlying comorbidities and his vital signs were within normal limits.
Initial treatment consisted of ulcer debridement followed by KERRAMAX CARE™ Super-Absorbent Dressing and a multilayered compression wrap. The dressing was changed 3 days later and revealed remarkable improvement. The ‘puddling’ of fluid was now completely abated and copious drainage had dramatically decreased (Figure 6).
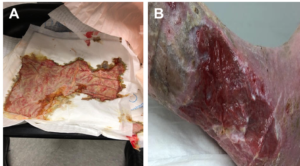
This therapy was continued with weekly dressing changes. After 1 month, the ulcer had healed, and the edema almost completely resolved (Figure 7).
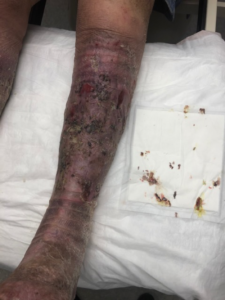
A support garment was prescribed, and pneumatic compression was ordered for daily use. A lymphedema therapist was recommended, and the patient has done well with only mild exacerbation of symptoms.
Patient data and photos courtesy of Robert Snyder, DPM, MSc, CWS.
As with any case study, the results and outcomes should not be interpreted as a guarantee or warranty of similar results. Individual results may vary depending on the patient’s circumstances and condition.
NOTE: Specific indiciations, contraindications, warnings, precautions and safety information may exist for these products. Please consult a healthcare provider and product instructions for use prior to application. Rx only.
References
- Moore JE, Jr., Bertram CD. Lymphatic System Flows. Annual Review of Fluid Mechanics 2018;50:459-482. doi:10.1146/annurev-fluid-122316-045259.
- Greene AK. Epidemiology and morbidity of lymphedema. In: Greene AK, Slavin SA, Borson H, eds. Lymphedema: Presentation, Diagnosis, and Treatment. 1st ed. Switzerland: Springer International Publishing; 2015:33-44.
- Kerchner K, Fleischer A, Yosipovitch G. Lower extremity lymphedema update: pathophysiology, diagnosis, and treatment guidelines. J Am Acad Dermatol 2008;59(2):324-331.
- Cormier JN, Askew RL, Mungovan KS, Xing Y, Ross MI, Armer JM. Lymphedema beyond breast cancer: a systematic review and meta-analysis of cancer-related secondary lymphedema. Cancer 2010;116(22):5138-5149. doi:10.1002/cncr.25458.
- Bakar Y, Tugral A. Lower Extremity Lymphedema Management after Gynecologic Cancer Surgery: A Review of Current Management Strategies. Ann Vasc Surg 2017;44:442-450. doi:10.1016/j.avsg.2017.03.197.
- Farrow W. Phlebolymphedema-a common underdiagnosed and undertreated problem in the wound care clinic. Journal of the American College of Certified Wound Specialists 2010;2(1):14-23. doi:10.1016/j.jcws.2010.04.004.
- Rockson SG. Diagnosis and management of lymphatic vascular disease. J Am Coll Cardiol 2008;52(10):799-806. doi:10.1016/j.jacc.2008.06.005.
- Morgan PA, Moffat CJ. International consensus on managing lymphoedema. Nurs Times 2006;102(44):42, 44.
- Torres Lacomba M, Yuste Sanchez MJ, Zapico Goni A et al. Effectiveness of early physiotherapy to prevent lymphoedema after surgery for breast cancer: randomised, single blinded, clinical trial. Br Med J 2010;340:b5396. doi:10.1136/bmj.b5396.
- Devoogdt N, Christiaens MR, Geraerts I et al. Effect of manual lymph drainage in addition to guidelines and exercise therapy on arm lymphoedema related to breast cancer: randomised controlled trial. Br Med J 2011;343:d5326. doi:10.1136/bmj.d5326.
- De Groef A, Van Kampen M, Dieltjens E et al. Effectiveness of postoperative physical therapy for upper-limb impairments after breast cancer treatment: a systematic review. Arch Phys Med Rehabil 2015;96(6):1140-1153. doi:10.1016/j.apmr.2015.01.006.
- Doscher ME, Herman S, Garfein ES. Surgical management of inoperable lymphedema: the re-emergence of abandoned techniques. J Am Coll Surg 2012;215(2):278-283. doi:10.1016/j.jamcollsurg.2012.03.020.
- Yan A, Avraham T, Zampell JC, Aschen SZ, Mehrara BJ. Mechanisms of lymphatic regeneration after tissue transfer. PLoS ONE 2011;6(2):e17201. doi:10.1371/journal.pone.0017201.
© 2020 3M. All rights reserved. 3M and the other marks shown are marks and/or registered marks. Unauthorized use prohibited. PRA-PM-US-02628 (07/20).

