
Dr. Robert Klein completed podiatric medical school in Chicago at the Rosalind Franklin University of Medicine and Science, Scholl College of Podiatric Medicine. Dr. Klein continued his surgical training as the Chief Resident at Michigan Health Center in Detroit. He is a Clinical Assistant Professor in the Department of Surgery at the University of South Carolina School of Medicine (USCSOM) Greenville and specializes in wound care, limb preservation, and Dr. Klein is a consultant for 3M.
Klein_Current-Dialogues-in-Wound-Management_2021_Article_6
Venous leg ulcers (VLUs) are a very challenging condition for clinicians and wound care providers to treat. Venous ulceration is a common skin and lower extremity problem that affects roughly 1% of the population and approximately 3-8% of people 80 years or older.1-4 The condition is a complication of chronic venous disease resulting from venous valvular incompetence and venous hypertension. Contributing factors for VLUs include inactivity, obesity, trauma/injury, diabetes, vasculitis, neoplasia, and older age.5 Outcomes for patients suffering from VLU are improved when the patient receives multidisciplinary care along with evidence-based wound care management.6,7 VLUs are a very common condition treated in the wound care center as well as by health care providers and clinicians in podiatry, family practice, geriatrics, physical therapy, general surgery, and vascular surgery settings.
Clinical Presentation and Diagnosis
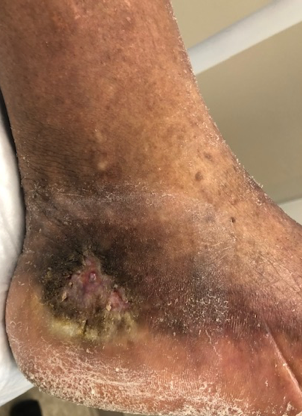
Venous ulcerations are typically located on the gaiter region of the lower leg, the area of the lower leg that is routinely covered by a sock. Common areas where VLUs are located include the medial aspect of the lower leg, medial ankle, and near the medial malleolus and can also be seen on the lateral lower leg and can also occur on the foot.8 On physical examination, venous ulcers are typically shallow and irregular in shape. Granulation tissue, fibrin, and exudate is commonly seen, as well as hemosiderin deposits, venous stasis dermatitis, and hyperpigmentation of the skin (Figure 1).
Appropriate diagnosis of a VLU is essential. Other types of ulcerations can occur on the lower extremity and/or foot, so understanding the clinical features of VLUs is an important step in their management. Common lower extremity and foot ulcerations include venous ulceration, arterial ulceration, pressure ulcerations, and neuropathic ulcerations
(Table 1).
Treatment
It is recommended that clinicians follow an evidence-based treatment approach for VLUs in which closure of the wound is the primary goal. The clinician should also formulate a treatment plan that prevents recurrence of the VLU once the wound is closed (e.g., compression hose and/or compression garments). The patient may require a vascular consult to treat the underlying condition.
Compression therapy is the standard of care for VLUs and it has been shown that venous ulcers heal more quickly when compression therapy is used.9 Compression therapy helps to reduce lower leg edema, addresses venous reflux, aids in wound healing, and helps reduce the pain associated with venous ulcers.10 There are many options available to the clinician when choosing compression therapy including one layer to various layers of compression.11,12 Using a multi-layer compression system that can provide high compression (35-40 mmHg) for patients with an ankle brachial pressure index (ABPI) ≥ 0.8 (3M™ Coban™ 2 Two-Layer Compression System), and reduced compression (25-30 mmHg) for patients with an ABPI ≥ 0.5 (3M™ Coban™ 2 Lite Two-Layer Compression System) may provide clinical benefit. These multi-layer compression systems provide sustained pressure without slipping, reduces edema and leg pain, and supports healing.13,14 This multi-layer compression system is also cost effective, providing sustained, therapeutic compression for up to 7-10 days.
Advanced wound care dressings (AWDs) are also part of the evidence-based treatment approach for VLUs. AWDs are often used under compression therapy to promote an environment conducive to wound healing. Using a collagen matrix with oxidized regenerated cellulose (ORC) and silver-ORC (3M™ Promogran Prisma™ Matrix) may help in this regard. In the presence of wound exudate, Promogran Prisma Matrix transforms into a conformable gel allowing contact with all areas of the VLU. The silver-ORC in the wound matrix serves as an antibacterial barrier and has been shown to reduce bacterial growth in vitro of common wound pathogens including Staphylococcus aureus, Pseudomonas aeruginosa, Eschericia coli, and Streptococcus pyogenes.15 The ORC component has been reported to lower the pH and reduce protease and matrix metalloproteinase levels which can be elevated in chronic wounds like VLUs.16,17
Wound bed preparation helps with wound healing. Debridement of necrotic and non-viable tissue has been shown to reduce wound size in comparison to patients whose wounds were not treated with debridement.18 There are multiple options available for wound debridement including biologic, autolytic, enzymatic, mechanical, ultrasonic, and surgical debridement.
VLUs can produce high amounts of exudate. Managing exudate while also protecting the integrity of the surrounding skin is an important component of the treatment plan. Clinicians may consider a non-cytotoxic, hypoallergenic no sting barrier film (3M™ Cavilon™ No Sting Barrier Film) to protect the peri-wound. Additionally, selecting a primary dressing (such as 3M™ Tegaderm™ High Performance Foam Non-Adhesive Dressing or 3MTM TegadermTM Silicone Foam Non-Bordered Dressings) that can handle a high level of exudate and maintain an optimal wound environment can help minimize dressing changes, overall cost of care, and discomfort to the patient.
Case presentation
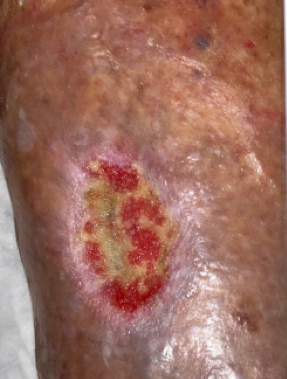
A 76-year-old female presented with a left leg wound (Figure 2) that had been present for more than 3 months and was unresponsive to treatment by her primary care provider. The patient’s previous care consisted of weekly applications of an Unna boot. Her past medical history was significant for coronary artery disease, hypertension, dyslipidemia, bronchiectasis, and chronic venous insufficiency. Examination of the left lower leg revealed dense hyperpigmentation from the midcalf down to her left foot. Reticular and spider veins were noted on her ankle and foot.
A 4-mm punch biopsy was performed as the wound had been present for at least 3 months with no significant healing. The pathology report revealed benign skin with ulceration, suppurative inflammation and prominent stasis changes; no evidence of malignancy was noted. After the wound evaluation and the biopsy results, the wound was diagnosed as a VLU.
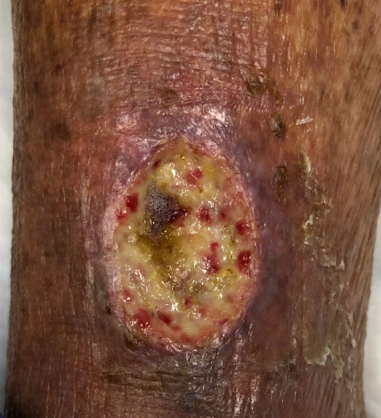
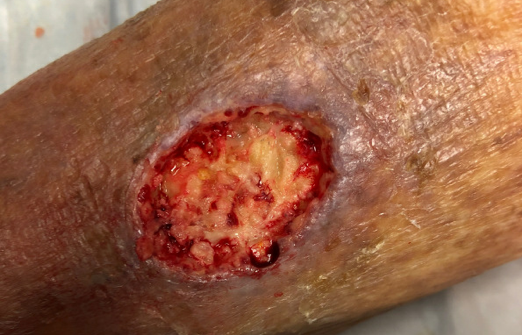
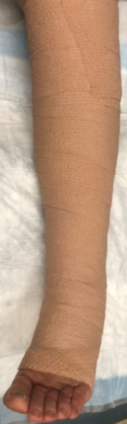
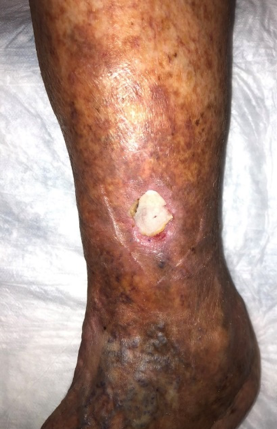
At the patient’s next visit, wound debridement was performed (Figure 3), followed by application of Tegaderm High Performance Foam Non-Adhesive Dressing as the primary dressing, and compression therapy with the Coban 2 Lite Compression System with dressing changes scheduled twice a week (Figure 4). Promogran Prisma Matrix was added to the patient’s treatment plan at week 3 (Figure 5). The patient’s wound improved considerably since her initial presentation, and at week 6 there was a notable decrease in the size and depth of the wound with robust granulation tissue present (Figures 6,7). At the wound care center, the goal is to achieve wound closure within 12 weeks of presentation. This patient is currently undergoing treatment and the wound is continuing to heal with decreasing wound size measured at each dressing change. Upon closure, a compression garment will be prescribed to help prevent reoccurrence and the development of new ulcerations.

CONCLUSION
This paper described an evidence-based approach for the treatment of venous leg ulceration. In patients with VLUs, a vascular consult to treat possible underlying conditions should be performed and may help prevent VLU reoccurrence. Additionally, wound debridement, advanced wound care dressings, and compression therapy use can help close these difficult wounds. Once closed, lifelong treatment with support hose and compression garments can help prevent recurrence.
References
1. Posnett J, Gottrup F, Lundgren H, Saal G. The resource impact of wounds on health-care providers in Europe. J Wound Care. 2009;18(4):154-161.
2. Bergan JJ, Schmid-Schonbein GW, Smith PD, Nicolaides AN, Boisseau MR, Eklof B. Chronic venous disease. N Engl J Med. 2006;355(5):488-498.
3. Jankunas V, Rimdeika R, Jasenas M, Samsanavicius D. Changes in patient’s quality of life comparing conservative and surgical treatment of venous leg ulcers. Medicina. 2004;40(8):731-739.
4. Moffatt CJ, Franks PJ, Doherty DC, Martin R, Blewett R, Ross F. Prevalence of leg ulceration in a London population. QJM. 2004;97(7):431-437.
5. Simon DA, Dix FP, McCollum CN. Management of venous leg ulcers. BMJ. 2004;328(7452):1358-1362.
6. Morrell CJ, Walters SJ, Dixon S, et al. Cost effectiveness of community leg ulcer clinics: randomised controlled trial. BMJ. 1998;316(7143):1487-1491.
7. Simon DA, Freak L, Kinsella A, et al. Community leg ulcer clinics: a comparative study in two health authorities. BMJ. 1996;312(7047):1648-1651.
8. Hafner J, Ramelet AA, Schmeller W, Brunner UV. Management of leg ulcers. Curr Probl Dermatol. 1999;27:4-7.
9. O’Meara S, Cullum NA, Nelson EA. Compression for venous leg ulcers. Cochrane Database Syst Rev. 2009(1):CD000265.
10. Nair B. Compression therapy for venous leg ulcers. Indian Dermatol Online J. 2014;5(3):378-382.
11. Fletcher A, Cullum N, Sheldon TA. A systematic review of compression treatment for venous leg ulcers. BMJ. 1997;315(7108):576-580.
12. Hettrick H. The science of compression therapy for chronic venous insufficiency edema. J Am Col Certif Wound Spec. 2009;1(1):20-24.
13. Mosti G, Crespi A, Mattaliano V. Comparison Between a New, Two-component Compression System With Zinc Paste Bandages for Leg Ulcer Healing: A Prospective, Multicenter, Randomized, Controlled Trial Monitoring Sub-bandage Pressures. Wounds. 2011;23(5):126-134.
14. Moffatt C, Edwards L, Collier M, et al. A randomized controlled 8-week crossover clinical evaluation of the 3M Coban 2 Layer Compression System versus Profore to evaluate the product performance in patients with venous leg ulcers. Int Wound J. (2):267-279.
15. Wu S, Applewhite AJ, Niezgoda J, et al. Oxidized regenerated cellulose/collagen dressings: review of evidence and recommendations. Adv Skin Wound Care. 2017;30(11S):S1-S18.
16. Masci E, Faillace G, Longoni M. Use of oxidized regenerated cellulose to achieve hemostasis during laparoscopic cholecystectomy: a retrospective cohort analysis. BMC Res Notes. 2018;11(1):239.
17. Cullen B, Watt PW, Lundqvist C, et al. The role of oxidised regenerated cellulose/collagen in chronic wound repair and its potential mechanism of action. Int J Biochem Cell Biol. 2002;34(12):1544-1556.
18. Bonkemeyer Millan S, Gan R, Townsend PE. Venous Ulcers: Diagnosis and Treatment. Am Fam Physician. 2019;100(5):298-305.
Patient data and images courtesy of Robert J. Klein, DPM, FACFAS, CWS.
As with any case study, the results and outcomes should not be interpreted as a guarantee for warranty of similar results. Individual results may vary depending on the patient’s circumstances and condition.
NOTE: Specific instructions, contraindications, warnings, precautions, and safety information exist for these products and therapies, some of which may be Rx only. Please consult a clinician and product instructions for use prior to application.
© 2021 3M. All rights reserved. 3M and the other marks shown are marks and/or registered marks. Unauthorized use prohibited. Used under license in Canada. PRA-PM-US-03000 (03/21).

