Dr. Orgill is Vice Chairman for Quality Improvement in the Department of Surgery at Brigham and Women’s Hospital and Professor of Surgery at Harvard Medical School. He is a reconstructive plastic surgeon and has a PhD from MIT in Medical Engineering. He is the Director of the Brigham and Women’s Hospital Wound Care Center and runs a tissue engineering and wound healing Laboratory. His lab at BWH is working to develop better technologies to treat wounds including work with artificial skin, micromechanical forces, platelets and stem cells. He has consulted for several medical device and start-up companies and is the inventor on several patents. He worked on the team that developed Integra, a skin replacement therapy that has been commercially developed and used successfully on thousands of patients.
Orgill_Current Dialogues in Wound Management_2015_Volume 1_Issue 1
INTRODUCED by KCI in 1995, Vacuum Assisted Closure® (V.A.C.®) devices have had an impact on the healing of wounds. Drs. Louis Argenta and Michael Morykwas from Wake Forest University had worked for years on several prototype devices where suction was used to facilitate wound closure. This included devices that used a CPR mask to close the wound and connect it to suction. One of the devices they found most promising was when they used a highly porous polyurethane foam beneath a semi-occlusive dressing and connected this to suction. My colleague, Karl Breuing, MD, introduced this to our hospital (Brigham and Women’s Hospital) at that time and had some amazing results. Soon after, the other plastic surgeons in our hospital, followed by surgeons of other disciplines, adopted this remarkable technology.
We first noticed a robust granulation tissue that formed when the device was placed onto wounds. The appearance of this seemed to mimic the porosity of the surface of the polyurethane foam. We wondered how this worked. As we asked colleagues, most people felt that it was because we were removing fluid from the wound and taking the “bad stuff” out. We noticed this same response in several other wounds where there was minimal fluid drainage and wondered if there was another mechanism involved
Quentin Eichbaum, MD, PhD, was a third-year medical student at Harvard Medical School and saw a patient (on whom he needed to do a report) with a V.A.C.® Therapy device. He put together a team from Harvard Medical School, including Donald Ingber, MD, PhD, and some MIT graduate students, to propose additional mechanisms to explain what we were seeing in the clinic. We constructed a finite element model of the wound and its interaction with the foam. The model predicted that the foam would deform the wound, and, according to previous work of Dr. Ingber, this deformation would also deform cells, prompting them to divide and proliferate. This theory we refer to as microdeformation.
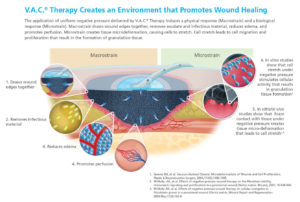
Many had also noted that these devices draw the wound edges together; this is referred to as macrodeformation. Others have proposed that keeping a wound sealed allows it to be warm and moist, which is well known to contribute to wound healing.
Undoubtedly, there are several mechanisms at work in the application of V.A.C.® Therapy devices. We and many others are in the process of unraveling the mechanism of these devices with the hope that this improved understanding will lead to better application of the existingdevices and provide clues as how to better design improved devices in the future.
In summary, we believe that six primary mechanisms of V.A.C.® Therapy devices are: 1) macrodeformation; 2) microdeformation; 3) removal of fluids; 4) reduction of edema, 5) promotion of wound bed perfusion, and 6) keeping the wound covered so it maintains constant moisture content. We look forward to learning much more about these mechanisms in the future.
References
- Saxena V, Hwang CW, Huang S, Eichbaum Q, Ingber D, Orgill DP. Vacuum-Assisted Closure: Microdeformations of Wounds and Cell Proliferation. Plast. Reconstr. Surg. 2004;114:1086-1096.
- Snyder RJ, Driver V, Fife C, et al. (2011) Using a diagnostic tool to identify elevated protease activity levels in chronic and stalled wounds: a consensus panel discussion. Ostomy Wound Management. 57(12): 36-46