D. Heath Stacey, MD is a plastic surgeon in private practice in Northwest Arkansas. His clinical interests include breast reconstruction, complex abdominal reconstruction, lower extremity reconstruction, breast and body contouring and facial aesthetic surgery.
Graftjacket® regenerative tissue matrix (RTM) Case Series
CHRONIC lower extremity wounds represent a clinical challenge that affects 6 million people per year. Bioengineered skin substitutes for full thickness skin wounds may achieve faster healing than local wound care alone and provide a solution which does not require a skin graft donor site.
Acellular dermal matrices1-4 may be used to promote wound healing in a wound bed that is well vascularized and has had previous devitalized/infected tissue debrided.
This cases series represents a diverse class of wounds ranging from venous insufficiency to traumatic to diabetic ulcers.
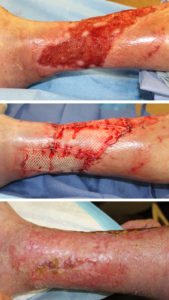
The patient in Figure 1 is a 72 y/o male with a history of chronic venous insufficiency of the lower extremities with a large lower extremity wound. He was on chronic immunosuppression for a renal transplant and had this wound for over 1 year before treatment. He underwent Graftjacket® regenerative tissue matrix (RTM) placement, which led to wound closure after two grafting sessions. The total wound measurements were 330 cm2, and he maintains stable closure at 2 years.
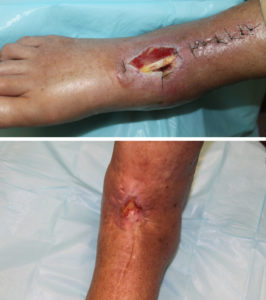
The patient in Figure 2 is an 83 y/o female with diabetes mellitus and peripheral vascular disease referred with a post-surgical dorsal foot wound after an ankle fusion. The wound contained exposed tendon and, given her vascular disease, she was not a candidate for vascularized tissue transfer/rotation flap. She was treated with Graftjacket® RTM placement and NPWT to cover the 4 cm2defect.
There are several ways of handling acellular dermal matrices intraoperatively and postoperatively. I have found that rehydration for at least 5 minutes in a solution containing 50,000 units of Bacitracin to be beneficial.
If one is having difficulty identifying the dermal side of the matrix, placing the matrix on the granulating wound to absorb blood will reveal the shiny dermal side before grafting. I place the graft under minimal tension and apply surgical staples to the wound edges.
Areas of tenting of the graft in large wound can be addressed by placing a 4-0 chromic interrupted suture. Overlapping of the graft over the wound edges may be done if adequate matrix is available.
To promote adherence of the matrix, I apply negative pressure wound therapy for 1 week with Bacitracin ointment and Adaptic® (Systagenix Wound Management, Limited) beneath the black foam sponge. The negative pressure is kept at 125mmHg. Compression with ACE™ Wraps (3M Company) and splinting to prevent motion during this first week are also important. The NPWT is removed at one week. If areas of the graft are not adherent, or there is minimal vascular ingrowth, the NPWT may be continued for another week. If the graft is taking well, then dressing changes with mineral oil, Adaptic® and compression are applied daily.
NOTE: As with any case study, the results and outcomes should not be interpreted as a guarantee or warranty of similar results. Individual results may vary depending on the patient’s circumstances and condition.
References
1. Reyzelman A, Crews RT, Moore JC, et al. Clinical effectiveness of an acellular dermal regenerative tissue matrix compared to standard wound management in healing diabetic foot ulcers: a prospective, randomised, multicentre study. Int Wound J 2009 June 1;6(3):196-208.
2. Brigido SA. The use of an acellular dermal regenerative tissue matrix in the treatment of lower extremity wounds: a prospective 16-week pilot study. Int Wound J 2006 September 1;3(3):181-7.
3. Brigido SA, Boc SF, Lopez RC. Effective management of major lower extremity wounds using an acellular regenerative tissue matrix: a pilot study. Orthopedics 2004 January 1;27(1 Suppl):s145-s149.
4. Winters CL, Brigido SA, Liden BA, et al. A multicenter study involving the use of a human acellular dermal regenerative tissue matrix for the treatment of diabetic lower extremity wounds. Adv Skin Wound Care 2008 August 1;21(8):375-381.
For complete product details and safety information, please visit www.kcielabeling.com.