
After completing his medical degree at Tulane University School of Medicine, Dr. Leong completed a general surgery residency at the Irvine Medical Center of the University of California and an internal medicine residency at New England Deaconess Hospital in Boston, Massachusetts. Fellowships in surgical oncology at the National Cancer Institute, National Institutes of Health; in medicine at Harvard Medical School; and in surgical oncology and research at Tulane University School of Medicine completed his training. Today, Dr. Leong focuses his practice on melanoma, cancer surgery, cutaneous oncology, breast cancer, and cancer research at the Sutter Pacific Medical Foundation at California Pacific Medical Center.
Leong_Current Dialogues in Wound Management_2015_Volume 1_Issue 4
INTRODUCTION
Surgical management of primary melanoma of the extremities can be challenging, as about 25 to 30% of patients develop regional lymph node metastases, necessitating radical lymph node dissection. Patients with metastatic melanoma of the lower extremity may be even more challenging to manage, as the anatomy of the groin is more complex than that of the axilla, making inguinal dissection more complicated than axillary dissection.
PATIENT HISTORY
A 68-year-old retired male police officer with a history of primary melanoma (0.7 mm thick) on the left arm was diagnosed on August 27, 2009, with a small area of regression for which he underwent re-excision with negative margins. He developed a second primary melanoma of 3.2 mm on the right leg following a biopsy on October 22, 2013. He underwent re-excision requiring a skin graft and had a negative right groin sentinel lymph node biopsy. He subsequently developed three papular lesions on the right posterior medial mid-calf with biopsy of these lesions on May 1, 2014. The biopsy revealed metastatic melanoma with dermal proliferation of atypical melanocytes. Molecular testing revealed the presence of the wild-type BRAF gene. Positron emission tomography–computed tomography (PET-CT) on May 20, 2014 found no definite evidence of metastatic disease, a stable hypermetabolic focus in the left ilium, and a hypermetabolic lesion in the left lobe of the thyroid.
His past medical history included hypertension; hypercholesterolemia, hypothyroidism, hypercalcemia, degenerative disc disease, prediabetes, basal cell carcinoma, and chronic pain. His past surgical history included partial thyroidectomy and parathyroidectomy, multiple neck and back surgeries, a carpal tunnel procedure, tonsillectomy, deviated septum repair, lumbar laminectomy, and hernia repair.
PRESENTATION AND INITIALSURGERY
The patient was seen in late May 2014, and as positron emission tomography-computed tomography (PET-CT) had revealed no systemic disease, surgical management was appropriate. The surgical recommendation was for excision of the in-transit biopsy sites on the right posterior leg with a 1-cm free margin, with possible use of a skin graft; sentinel lymph node mapping, and selective sentinel lymph node dissection.
At the preoperative visit on June 25, 2014, the patient mentioned three new 3-mm intracutaneous pink nodules in the right posterior mid-calf that had appeared within the past week. No adenopathy was palpable. On June 25, 2014, preoperative lymphoscintigraphy with Lymphoseek® (Navidea Biopharmaceuticals, Inc.) showed at least two channels leading to a distal femoral sentinel lymph node and proximal inguinal sentinel lymph nodes at the level of the inguinal ligament, but superficial to the external oblique aponeurosis. The patient was transferred to the operating room and the following procedures were performed under general anesthesia: wide local full-thickness re-excision of the previous biopsy scars of the in-transit metastatic melanoma in the right posterior medial mid-calf, full-thickness excision of three in-transit intracutaneous metastatic melanoma lesions in the right posterior medial mid-calf, development of 2-cm circumferential advancement flaps for primary closure of the wide local re-excision wound, and dissection of the right deep femoral and inguinal selective sentinel lymph nodes with intraoperative wireless Neoprobe® (Mammotome, a division of Devicor® Medical Products, Inc.) lymphatic mapping. Histopathology found the distal femoral sentinel lymph node, one of three femoral sentinel lymph nodes, with a 1.5-mm micrometastasis.
The case was suggestive of a high risk of recurrence in this patient.
SECOND SURGERY
The patient was therefore admitted on July 23, 2014 for a right radical ilioinguinal lymph node dissection, including suprainguinal, femoral and pelvic lymph nodes. Significant induration was found in the proximal femoral triangle over a 6 cm area, and it was resected along with the surprainguinal and femoral nodal basins, followed by deep pelvic lymph node dissection. Drains were placed and the wound was closed. All 12 lymph nodes removed were negative for metastatic melanoma. Following this procedure, the patient developed wound breakdown due to infection (Figure 1). Infection developed because the warm and moist environment of the groin allows bacterial growth. Drainage was copious, requiring several dressing changes a day.
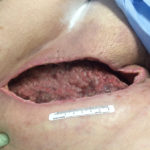 Figure 1. Initial wound appearance (20 cm x 10 cm x 6 cm)
Figure 1. Initial wound appearance (20 cm x 10 cm x 6 cm)
WOUND MANAGEMENT
In addition to the use of systemic antibiotic therapy, local management options to deal with the wound infection included taking the patient back to the operating room for debridement under general anesthesia. The decision was made that V.A.C.® Therapy would be the best way to manage this wound, and an ActiV.A.C.® Therapy Unit was placed on August 8, 2014
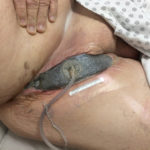 Figure 2. An ActiV.A.C.® Therapy Unit was placed initially on August 8, 2014
Figure 2. An ActiV.A.C.® Therapy Unit was placed initially on August 8, 2014
(Figure 2). The patient was initially seen weekly in the Melanoma Clinic and then monthly for evaluation of wound healing. In off weeks, dressing changes were performed at a local wound care clinic. Dressings were changed three times a week.
During the initial discussion and placement of V.A.C.® Therapy, the patient was apprehensive about the treatment. Two days after initial placement, he was seen in the Melanoma Clinic for follow up and was thrilled with the treatment. He was especially thrilled with having the copious drainage contained in a canister, which allowed him to complete his daily activities more easily. Reducing dressing changes to three times a week from three times a day allowed the patient and his wife to return to a more normal routine. Excellent development of granulation tissue was noted by the end of the first week (Figure 3). Use of the ActiV.A.C.® Therapy Unit was discontinued on September 17, 2014, after 5 weeks of NPWT, and the wound was sutured (Figure 4). By this time, the patient was also officially in remission from the melanoma. The local wound care clinic performed dressing changes until the wound healed completely, 3 months after discontinuation of NPWT. On August 10, 2015, long-term follow-up demonstrated complete resolution with some scarring, but little risk of skin breakdown or reopening.
 Figure 3. The wound shows signs of healthy granulation tissue formation and increased perfusion 1 week after initiation of V.A.C.® Therapy (August 15, 2014).
Figure 3. The wound shows signs of healthy granulation tissue formation and increased perfusion 1 week after initiation of V.A.C.® Therapy (August 15, 2014).
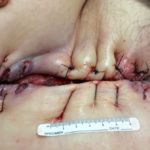 Figure 4. Use of the ActiV.A.C.® Therapy Unit was discontinued on September 17, 2014 after 5 weeks of treatment. The surgeon sutured the wound in preparation for final wound closure, and the patient’s local wound care clinic performed standard dressing changes until the wound healed completely.
Figure 4. Use of the ActiV.A.C.® Therapy Unit was discontinued on September 17, 2014 after 5 weeks of treatment. The surgeon sutured the wound in preparation for final wound closure, and the patient’s local wound care clinic performed standard dressing changes until the wound healed completely.
In this case, the use of V.A.C.® Therapy led to wound closure (Figure 5), while saving the patient several surgical procedures and general anesthetics, improving his quality of life, and reducing healthcare costs.
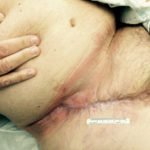 Figure 5. Complete wound closure
Figure 5. Complete wound closure
NOTE: As with any case study, the results and outcomes should not be interpreted as a guarantee or warranty of similar results. Individual results may vary depending on the patient’s circumstances and condition.
References
Mozzillo N, Caracò C, Marone U, et al. Superficial and deep lymph node dissection for stage III cutaneous melanoma: clinical outcome and prognostic factors. World J Surg Oncol. 2013;11:36. doi: 10.1186/1477-7819-11-36.

