Dr. Orgill is Vice Chairman for Quality Improvement in the Department of Surgery at Brigham and Women’s Hospital and Professor of Surgery at Harvard Medical School. He is a reconstructive plastic surgeon and has a PhD from MIT in Medical Engineering. He is the Director of the Brigham and Women’s Hospital Wound Care Center and runs a tissue engineering and wound healing laboratory. His lab at BWH is working to develop better technologies to treat wounds including work with artificial skin, micromechanical forces, platelets and stem cells. He has consulted for several medical device and start-up companies and is the inventor on several patents. He worked on the team that developed Integra®, a skin replacement therapy that has been commercially developed and used successfully on thousands of patients.
Orgill_Current Dialogues in Wound Management_2016_Volume 2_Issue 2
INTRODUCTION
With the increasing number of patients with wounds, the cost of wound care, coupled with the myriad of products available to treat wounds, it is no surprise that wound care treatments are coming under increased scrutiny. High levels of evidence are now required to help justify both the products and methods we use to treat complex wounds. The days when new products can be introduced based on a few anecdotal cases are quickly coming to an end. The government, insurance companies, healthcare providers and patients all want more rigorous clinical evidence to support the use of new products. Increasingly, there is a great interest in targeted products that focus on specific wound types and that have high quality evidence to back up their use. Here are some points wound care practitioners may wish to consider when evaluating a new product.
HOW WAS IT APPROVED THROUGH THE FDA?
All products in clinical use in the USA must be cleared for marketing or approved by the FDA. Other regulatory bodies approve products in other countries. Because there is a diversity of products used in wound care, there are very different pathways for products to be approved by the FDA. The FDA is best known for drugs that must be proven to be “safe and effective.” For new drugs targeted for widespread usage, this requires submission of a New Drug Application (NDA) with substantial pre-clinical and clinical studies, including properly designed prospective clinical trials. These studies can cost millions of dollars and, therefore, drug development is restricted to applications with a large market where the new drugs can be sold at a substantial premium. Considering the difficulty of getting a drug approved, the FDA has developed an Orphan Drug mechanism for drugs needed for rare diseases.
Many complex medical devices that promote active healing are approved through a Premarket Approval (PMA) Mechanism. These require in-depth clinical studies for safety and efficacy. In contrast, many wound products are now approved as medical devices that are substantially equivalent to those currently being used. This route is called a 510(k) premarket submission and may not require as much in the way of clinical studies. Although the FDA approval process is substantially similar to other products, many companies use other methods to distinguish new products from predicate devices.
Yet another mechanism of approval for human cells, tissues, or cellular or tissue-based products (HCT/Ps) is defined in the Code of Federal Regulations (CFR) Part 1271. Very often, these require strict sterility and tracking procedures, but frequently the clinical data required are less than that for a drug or a PMA device. Tissues or cells derived from the patient with “minimal manipulation” are not regulated by the FDA. What constitutes “minimal manipulation” is a subject currently being considered.
WHAT TYPES OF STUDIES HAVE BEEN DONE IN SUPPORT OF THE PRODUCT?
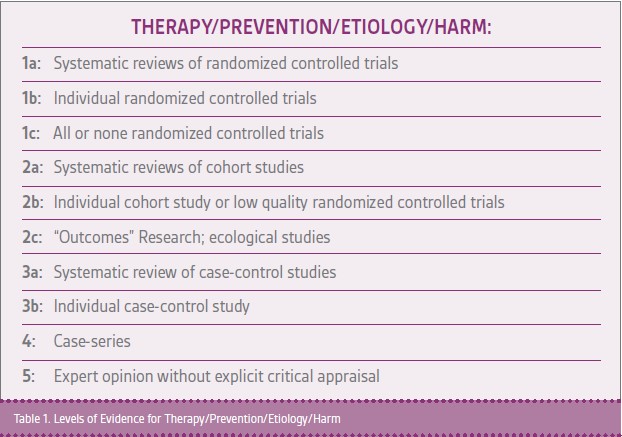
Clinical papers can be rated based on the level of evidence (Table 1). Generally, the highest quality studies are an aggregation of prospective randomized controlled trials (Level Ia studies) where both patients and clinicians are blinded to the types of treatment received and outcome measures are defined well in advance of the studies. Studies should be registered in advance of enrolling patients.
HOW MUCH BIAS IS THERE IN THE STUDY?
Biased studies can actually cause a lot of harm to patients. Experts that have looked at study bias have proposed several important checklists such as the CONSORT checklist that should be reviewed in assessing the quality of these trials.2 It is important to review these checklists both when designing and writing studies. Sometimes it is impossible to meet all of the guidelines due to the nature of treating wounds. For example, from a study perspective, it would be ideal to blind both the patient and physician to the type of dressing used but it may be impractical to do this for specific wound care treatments.
WHAT IS THE STUDY POPULATION?
In order to show a statistically significant difference between a treatment and a control arm, ideally the patients studied should be similar to those in the control arm. As a result, all studies have both inclusion and exclusion criteria that are applied in screening patients. Commonly, only a fraction of patients screened for a study are actually enrolled. So, if the study shows a treatment effect, clinicians should be cautious in applying this treatment to patients that would have been excluded from the reported randomized study. To better understand how a treatment works in a wider range of patients, many have turned to registry studies where all patients in which a particular product is used are closely followed.
WHO DID THE STUDY?
We all need to make judgments about the quality of the study based on the authors. Well-known authors who have a solid track record of publishing reputable studies are preferred. Studies written by companies that market products or those with authors who receive compensation from these companies should be reviewed with caution. Many wound care studies require physician-industry cooperation, but it is important to understand any potential conflicts of interest or commitment.
WHAT IS THE COST OF THE PRODUCT?
A great product with extensive peer review may not be successful in the market place if it is not reimbursed by insurance or is too expensive for the facility from a budget impact perspective. Providers need to consider the costs of products carefully in making decisions on what to use in particular patients.
CONCLUSIONS
Wound care is becoming increasingly complex with many exciting technologies coming to fruition. Evidence-based medicine will need to be applied to wound healing, as it is in many other fields, to best direct clinicians to the best drugs and medical devices to use. In addition, cost will become an increasingly important consideration as we face more demand for medical services in the coming years.
References
1.Agha RA, Orgill DP. Evidence-Based Plastic Surgery: Its Rise, Importance, and a Practical Guide. Aesthet Surg J. 2016 Jan 7. pii: sjv204. [Epub ahead of print]PubMed PMID: 26746230.
2.CONSORT Checklist. Consort-Statement. Available at http://www.consort-statement.org/checklists/view/32-consort/66-title. Accessed 1/11/2016.