
Ms. Beck graduated in 1997 with a BSN from Indiana University School of Nursing. She completed the Emory University Wound Ostomy and Continence Nurse Education Program and became certified in 2000. Ms. Beck worked as a WOC Nurse Clinician with Indiana University Health System in Indianapolis, Indiana from 1996-2014 and at Major Hospital, in Shelbyville, Indiana from 2014 to present.
Beck_Current Dialogues in Wound Management_2020_Article_3
During the early morning of February 2011, I was walking through the vacant halls of the academic health center where I work, on my way to my office. I spotted an environmental services technician working in the distance on the right, so I moved to the left. Before I knew it, my feet were completely out from under me, and I landed directly on my tailbone in wet wax. My pride and my rear end were both sore, but I pressed on to get my workday started.
In the fall of 2016, I noticed some tenderness and swelling between my anus and tailbone area. I consulted one of the surgeons between cases and told him about my problem. Following an examination, I was prescribed an antibiotic. After taking the medication as prescribed, the symptoms disappeared.
In October 2018, seemingly out of nowhere, I developed tenderness and swelling in the same area. I mentioned this to the same surgeon as well as to our newest general surgeon. I was examined and again prescribed an antibiotic. On day 6 of the antibiotic course, there was a rupture of purulent drainage. I immediately tried to examine the area myself without luck and I was forced to wait for an appointment. I went to a walk-in clinic and consulted with the nurse practitioner (NP) at my primary care physician’s office. After the examination, she believed that this may be a pilonidal cyst and recommended a general surgery consult.
With the consult in hand, I contacted my surgical colleague who had originally prescribed the antibiotics about this potential pilonidal cyst. The surgeon was concerned that this may be a perirectal abscess due to a history of constipation and anal fissures. He recommended an exam under sedation and if an abscess with fistula to the rectum was found, he wanted to perform a Seton procedure. Knowing a little bit about the Seton procedure, I researched perirectal abscesses and pilonidal cysts and wanted a second opinion.
I made an appointment with a plastic surgeon who treated pilonidal cysts. He ordered a computerized tomography (CT) scan to check for sinus tract and any evidence of inflammatory bowel disease (IBD). The CT was negative for abscess, sinus tract or IBD– so we were back to suspecting pilonidal cyst. The only risk factor present for pilonidal cyst was the injury to the tailbone from 2011. At this point in time, I had 2 small open areas that drained intermittently, and was not experiencing pain. I decided not to do anything and hoped that the sites would heal on their own.
A few months later, both open sites had become one open area. I contacted my surgeon and was scheduled for pilonidal cyst excision in January of 2019. The surgical procedure was confirmed to be for a pilonidal cyst with removal of debris and straightforward repair with clean wound edges. The surgeon opted to close the wound with internal absorbable sutures and external skin sutures. I was released to go back to work the next day.
On post-operative day (POD) 7 there was a slight dehiscence at the superior aspect of the incision. On POD 14, I had a follow up appointment and the sutures were removed. On POD 17, there was dehiscence of the incision.
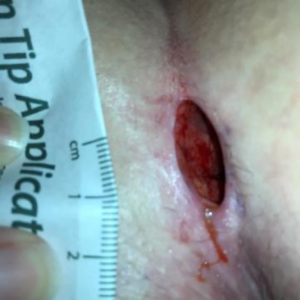
At my first visit to the wound clinic, my wound measured 2 cm x 1 cm x 1 cm full thickness to the subcutaneous tissue and the internal suture was visible ( Figure 1). A hydrofiber dressing was applied 3 times per week. However, the dressings were not staying on well, and daily dressing changes were needed. I had a hard time changing my own dressings and it occurred to me that the negative pressure wound therapy could be used if a seal could be achieved. I would also need to conceal the device as I wanted to be able to work while healing this wound. Since I see patients 3 days a week in an acute care hospital, I did not think patients would feel comfortable with me taking care of them while lugging around a large medical device. I also did not want to risk possible contamination of the wound, if dressing changes needed to occur while at work.
The disposable, non-powered SNAP™ Therapy System came to mind. I had not used the SNAP™ System in my practice and I contacted my local manufacturer’s representative to inquire about a product trial. I arranged to have the representative meet me at my next appointment.
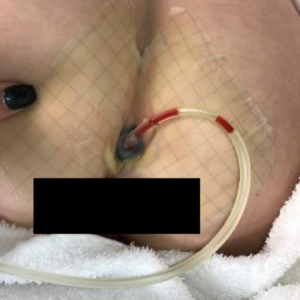
In February of 2019, the wound measured 2 cm x 0.7 cm x 1 cm on the day of application of the SNAP™ System. A protective barrier wipe was used to protect the periwound skin and a barrier ring was used to help maintain seal at the crease of the buttocks. Foam was packed into wound and port tubing pulled to the side to be out of the way for toileting. The plunger was used to activate -125 mmHg of negative pressure (Figures 2 and 3).
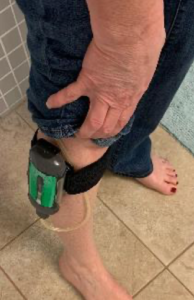
I was able to keep the device in my pocket or clip it onto the SNAP™ Therapy Strap on my leg. The SNAP™ System was small enough not to be noticeable under clothing (Figure 4). When I showered, I used a clip to hang the device on the shower curtain which held it away from the water and kept it dry (Figure 5). The dressing stayed sealed during everyday activities including showering and bowel movements. There was no noise with the device and no power cord, so sleep was not interrupted. The tubing was long enough to put on either side, depending on which side I was laying on. I was able to go about my usual activities without worrying about the device being visible, making noise, or needing to be charged.

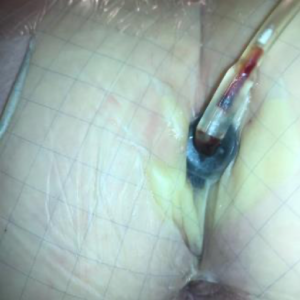
On Day 4, the dressing was changed by a fellow wound care nurse (Figure 6). The dressing change experience was more unpleasant than I expected, with the removal of the transparent film causing a burning sensation, like a sunburn, that lingered for about 30 minutes after the new dressing was placed, even with the use of an adhesive releaser. The removal of the foam was painful but only for a few seconds and then subsided. There had been some periwound skin exposed to the foam which had caused some periwound skin trauma that was tender with cleansing. Keeping the dressing in place and not having to change it more than twice a week was great and definitely worth the slight discomfort experienced at the dressing changes.
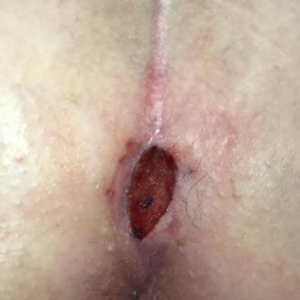
On Day 7 of using the SNAP™ System, my wound measured 2 cm x 0.5 cm x 0.6 cm, and the internal suture visible in the base of wound was removed. There was odor noted with the dressing change. A wound culture was taken followed by debridement of non-viable tissue and application of silver nitrate.
On Day 11, the wound measured 1.7 cm x 0.5 cm x 0.7 cm. There was no odor with my dressing change although cephalexin use was initiated due to a positive wound culture from my previous visit. The dressing remained in place and I removed the SNAP™ Therapy System, due to a trip out of the country because I wanted to avoid security concerns and/or dressing problems during the trip. I performed daily dressings during this time. After my trip, I returned to the wound clinic and my wound measured 1.5 cm x 0.5 cm x 0.6 cm and my dressing was replaced as before.
At my next wound clinic visit my wound measurements were 0.0 cm x 0.0 cm x 0.0 cm! I used a piece of silicone tape over the site for 3 weeks after my last visit to protect the wound from moisture and friction.
This experience helped me to better appreciate the patient journey as it related to dressing changes, odor, bathing, working, sleeping and healthcare provider visits in the context of wound care management. I have a new appreciation for what patients go through day-to-day. Having the SNAP™ System allowed me to discretely continue healing my wound while working and continuing with my usual daily activities. This personal and first-hand experience as a patient has also given me the confidence to recommend the SNAP™ System for pilonidal cyst wound treatment, for situations where wound size and drainage warrant use of a disposable device, or for scenarios in which the patient desires a quiet, non-powered device option.
As with any case study, the results and outcomes should not be interpreted as a guarantee or warranty of similar results. Individual results may vary depending on the patient’s circumstances and condition.
NOTE: Specific indications, contraindications, warnings, precautions, and safety information exist for KCI products and therapies. Please consult a clinician and product instructions for use prior to application. Rx only.
@Copyright 2020 3M. All rights reserved. 3M and the other marks shown are marks and/or registered marks. Unauthorized use prohibited. PRA-PM-US-02365 (04/20).
disposable negative pressure therapy, SNAP Therapy, chronic wound, pilonidal cyst.

