Dr. Lincoln is a full time Wound Care and Hyperbaric Medicine Medical Director in Lampasas, Texas with Central Texas Wound Healing Associates. She is a graduate of Siena College, Loudonville,NY with a BS Cum Laude in Chemistry, the University of New England College of Osteopathic Medicine for her DO degree, and Wilson Memorial Hospital Family Medicine residency. Dr Lincoln has been involved with various wound care publications and peer review journals. Dr Lincoln is very actively involved with severalTexas medical organizations, including currently serving as the President of Texas Society of the American College of Osteopathic Family Physicians.

Dr. Shaw graduated from Albright College in Reading, Pa. with a bachelor of science and a medical degree from Des Moines University in Des Moines, Iowa. He is board certified and a fellow in emergency medicine, He is also a fellow of the American Board of Wound Healing.
Today, Dr. Shaw is a medical director and full time clinician at the Mount Nittany Center for Wound Care in State College, Pa. with extensive experience in epidermal grafting having performed over 400 procedures. He currently functions as a consultant, national/regional speaker and advisory board member for the Acelity corporation.
Lincoln_Advanced Wound Dressings Today_2017_Spring Supplement
Chronic wounds are challenging for all wound care providers. Knowledge of wound care science has evolved significantly in the last 20 years. Now, providers of wound care have so many choices of materials and products, each with its own specific wound specific properties. As a patient travels through the wound treatment journey, the wound care providerisable to move through a continuum with the patient.² As the wound closes and changes, so should the products used to treat the wound.
In this article,two case reports will be reviewed,demonstrating the importance of choosing a product to address the state of the wound over a time period encompassing multiple weeks.
Case 1
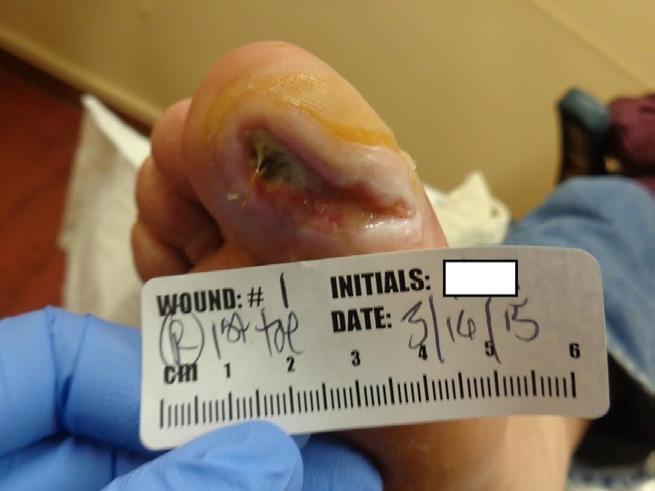
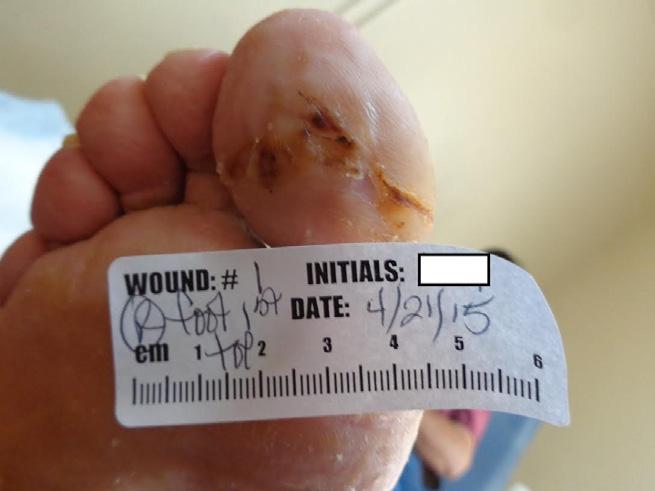
This 42 year old male with diabetes and diabetic neuropathy presents to the outpatient wound care center (WCC) with his right great toe on the plantar surface. His medical history is also significant for coronary artery disease and hypertension. The wound has been present for 5 months and the patient has completed a course of TMP/SMX (trimethoprim/sulfamethoxazole) from another provider without wound resolution. The wound measures 4cm x 2cm x 0.4cm at the initial assessment at the WCC. (Figure 1-A)
With the patient’s medical history in mind, an ankle-brachial index (ABI) was done in the office and showed a 0.9 ratio bilateral, suggesting reasonable arterial flow to this toe wound. After vascular assessment, the necrotic tissue was addressed. Some of the thick adherent, yellow fibrin was removed with scissor and forceps. The wound base could not clearly be seen and a topical collagenase ointment was chosen for topical debridement. Topical collagenase was added to the wound daily during this period. A total contact cast is the gold standard for a diabetic toe ulcer, but this patient refused application.1 A medical equipment shoe was chosen for off-loading, although this is neither ideal nor the preferred approach
The wound was cultured at the initial visit and was found to have no pathologic bacteria after 7 days. Diabetic foot wounds often develop infections so high clinical suspicion of infection should be maintained throughout the wound care journey.
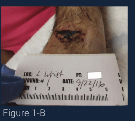
Figure 1-B shows the wound at one week into treatment at the WCC. Topical debridement was used daily for 1 week and one can note that the fibrin burden as well as the overall size of the wound has been significantly reduced. A curette was used to attempt removal of the remaining necrotic material. As there was still an adherent layer of fibrin covering the wound after debridement, topical collagenase was used daily for an additional week. This ointment is covered with a foam dressing and the patient is compliant with a medical equipment shoe.
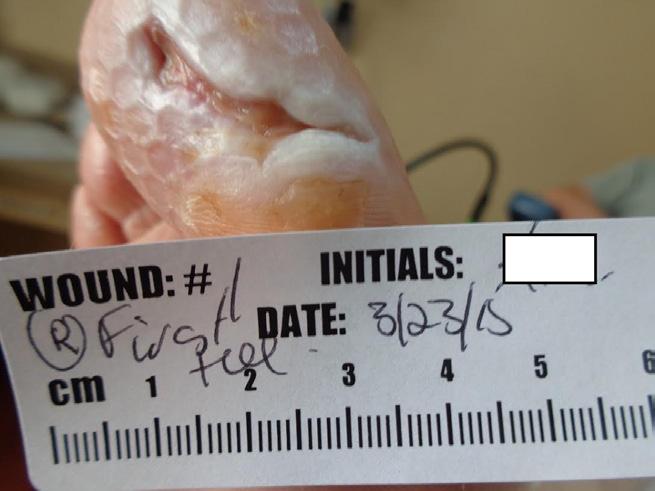
The week 3 image (Figure 1-C) reflects significant progression of this wound toward closure. There is a significant reduction of fibrin and the wound base shows improved granulation tissue. Curetting this wound revealed a red, beefy, granular base. A sterile, freeze dried mix of 44% oxidized regenerated cellulose (ORC), 55% collagen, and 1% silver-ORC was applied to the base.3 This combination product was chosen to maintain a physiologically moist microenvironment, leading to optimized wound healing. In addition, this product is chosen for its antimicrobial properties as diabetic foot wounds are at risk for polymicrobial colonization which can progress to frank infection.
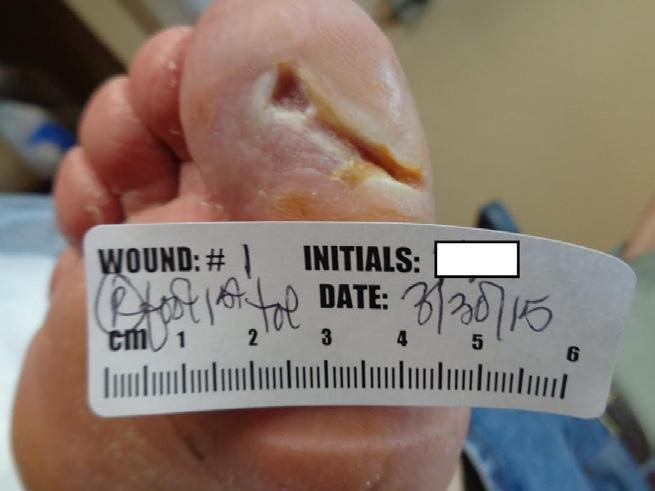
(Figure 1-D) This image shows a healing wound at week 6. The wound was able to overcome adversity, including off-loading in a secondary choice device due to patient demand. The matrix was able to provide a local microenvironment conducive to wound closure. At this discharge visit, the patient was extensively educated about the risk of diabetic foot ulcers, risk of amputation, and need for proper foot wear to avoid future ulcerations. Long term control of blood sugars has been emphasized. At discharge, the patient agrees to diabetic shoes as a preventative device.
Case 2
This 36 year old female with rheumatoid arthritis taking methotrexate presents with a surgical non-healing wound. Her other medical conditions include polycystic ovary syndrome (PCOS), hypothyroidism, and hypertension. The patient had left wrist surgery to remove a rheumatoid nodule, her sutures were removed at post-op day 10 and the wound was not closed. Sutures were replaced for 2 weeks and when they were removed, the wound continued to have a significant deficit in closure. The patient’s first visit to the WCC was at 4 weeks post-op revealing a non-healed surgical wound on her left wrist (Figure 2-A).
On presentation, the wound measured 0.6cm x 2.4cm x 0.8cm. When a portion of eschar was removed with scissor and forceps, a significant tissue defect was noted. Post-debridement the wound measured 1.0 cm x 2.5 cm x 0.9 cm with tendon exposed.4 After debridement, a wound culture was obtained from the base of the wound. Packing gauze was saturated with half strength hypochlorite solution (Dakins solution) and changed daily. It was chosen as a topical antiseptic while the culture was pending.
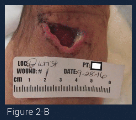
(Figure 2-B) At the one week interval, the wound base can be clearly seen. A selective debridement was performed in the office to remove dead tissue and slough, depicted in the photograph as yellow material. The base of the wound was prepared with a curette to remove biofilm and create a fresh surface. A freeze dried mix of 44% oxidized regenerated cellulose (ORC), 55% collagen, and 1% silver-ORC was applied to the base of the wound and wound was covered in a foam border dressing.5 An additional matrix was added three times a week. Light compression was applied with a tubular compressive dressing. The culture from presentation was reviewed and showed no pathologic growth.
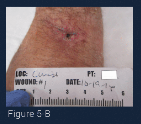
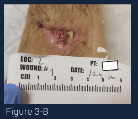
(Figure 3-B) At week 3 of care, this image shows a marked reduction in wound volume and improved quality of granulation tissue. The yellow necrotic tissue was removed with a curette and the wound was irrigated with saline. Additional ORC/collagen/silver was placed in the wound base and the wound was covered with a foam border dressing. Light compression was maintained.
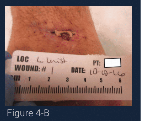
(Figure 4-B) At week 4 of care, a good wound closure trajectory is seen in this photograph. ORC/collagen/silver can be seen stuck to the wound surface. The wound was cleaned and a curette was used to remove all non-viable tissue. The base of the wound was at skin level and red/pink granulation was seen. ORC/collagen/silver was reapplied and compression resumed. No signs of infection or inflammation were observed and no significant discharge from the wound was noted. Additionally, there was no limitation in the range-of-motion of the joint. The patient continues to take daily methotrexate for rheumatoid arthritis.
(Figure 5-B) This patient is discharged at week 5 of care from the WCC with a healed surgical wound on the left wrist, after the small fragment of material was removed.
While chronic wounds are difficult to heal, advanced wound care products and dressings give providers the tools to heal these chronic wounds. As these cases demonstrate, products used to treat chronic wounds can be changed to adapt to the situation.
Patient data and photos courtesy of Katie Lincoln, DO, FAAFP; Texas, USA
NOTE: Specific indications, contraindications, warnings, precautions and safety information may exist for Systagenix products. Please consult with a healthcare professional and product instructions for use prior to application. Rx only.
Copyright 2017 Systagenix Wound Management, Limited. All rights reserved. All trademarks designated herein are proprietary to Systagenix Wound Management Limited, KCI Licensing, Inc., and their affiliates and/or licensors. PRA000310-R0-US, EN (03/17)
Wound Bed Preparation for Epidermal Grafting
NOTE: As with any case study, the results and outcomes should not be interpreted as a guarantee or warranty of similar results. Individual results may vary depending on the patient’s circumstances and condition.
By: Mark R. Shaw, DO, FACEP, FAPWCA
Today, after more than 400 epidermal grafts performed at our wound center, this procedure has become the treatment of choice in our center for the final closure of wounds and ulcers when indicated. With a success rate above 90%* we have learned a few things that help to make that a reality. Essential to that outcome is wound bed preparation.
Wound bed preparation is defined as “the management of the wound to accelerate endogenous healing or to facilitate the effectiveness of other therapeutic measures.” There are multiple factors that will ultimately affect the outcome of wound healing. Each one of these has to be appropriately addressed and managed in order to develop a wound bed that is adequately prepared for epidermal grafting. Not to ignore anyone of these elements; it has been our experience that there are 4 primary factors that have demonstrated the highest incidence for graft success. They are:
strated the highest incidence for graft success. They are:
1.Recognition of adequate blood flow
2.Development of an appropriate granulation wound bed
3.Infection control
4.Chronic inflammation management
Primary attention to these along with other exogenous factors will go a long way to achieving the ultimate result of closure.
All patients need appropriate vascular screening to assure that adequate oxygen at the tissue level is present. Patients with lower extremity wounds should have initial ankle brachial index evaluations for this purpose regardless of the type of wound being managed. If results indicate an increased probability of vascular insufficiency arterial duplex ultrasonography is performed and the patient sent for vascular consultation. If indicated, revascularization is performed prior to grafting. In our experience arterial ulcerations have the highest incidence of graft failure. Recognition and treatment therefore becomes essential in this class of wound.
* Success rate defined as complete closure in 12 or less weeks
An adequate granulation bed is the foundation needed for epidermal graft placement. It must be void of eschar, thick fibrinous exudate such as slough and necrotic tissue. Sequential sharp surgical debridements are paramount in meeting this end. This is performed on a weekly basis until only granulation tissue is present. Keep in mind that gentle management of tissues during this process will maintain and enhance neovascularization, the literal lifeblood of the graft. If the patient is unable to tolerate this form of debridement or an existing coagulopathy prohibits it, enzymatic debridement is a viable alternative. Ideally, all tendon or bone should be covered. Improving the wound depth is required to obtain a reasonable cosmetic outcome. Control of wound depth in many cases can be best managed with NPWT. Ultimately developing a depth of 2 mm or less prior to graft application will limit the deformity created by healing over an untoward cavitation.
Infection control requires early recognition and diagnosis. Obtaining a proper wound culture is the first step in this process. A deep tissue source following debridement is critical in evaluating the wound for an acute infection. Targeted antibiotic therapy can then proceed. The eradication of an active infection will reduce the significant potential for graft failure. We recommend a minimum of 2 weeks of treatment prior to grafting. Obviously, this can be longer when IV therapy or when multi organism infection control is required.
When a wound has developed a state of persistent inflammation a graft failure is almost assured. When a potential wound for grafting demonstrates this stalled status (no change in status over 2-4 weeks), one such cause can be increased activity of the inflammatory cells. We now use collagen with ORC as a primary dressing for 2 weeks prior to grafting in all patients. We believe that this helps to promote the progression of the wound from the inflammatory to the proliferative stage of wound healing which is more receptive to receiving an epidermal graft.
In order to help elucidate this process of wound bed preparation for epidermal grafting the following two clinical cases give a very good representation of affective outcomes when the factors that affect wound healing are controlled and appropriate interventions utilized.
CASE 1
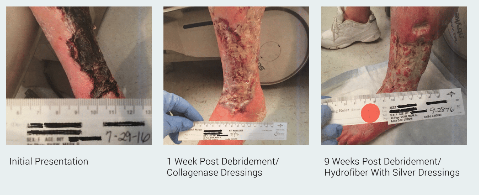
This case involves a 66 F with extensive tissue loss of the left lower extremity due to arterial ulcerations in the face of significant PVD. This patient has multiple co-morbid conditions including CAD, hypertension and a prior failed fem-pop bypass graft. She is still under our care having just received her first two staged epidermal grafts. Her treatment in the clinic has encompassed 6 months of therapy including surgical debridement, collagenase dressing, NWPT, IV and oral antibiotic therapy and endovascular revascularization.
CASE 2
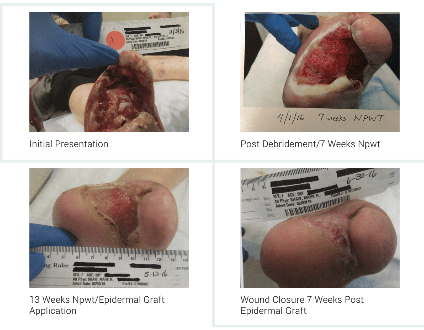
This case involves a 67 F who underwent a trans-metatarsal amputation of her left foot and additional surgical debridement for osteomyelitis and necrotizing fasciitis/gas gangrene. This patient has significant co-morbid conditions that consist of diabetic neuropathy, PVD, hypertension, hypothyroid disease and nicotine addiction. Her treatment in the clinic included surgical debridement, NWPT (13 weeks), HBO (60 treatments), IV and oral antibiotic therapy and epidermal grafting to closure.
CONCLUSIONS
In our opinion, epidermal grafting, in its current state, is becoming more and more recognizable as the standard of practice for the final closure procedure of any wound or ulcer. Its ease of application, patient safety, patient convenience and excellent closure results will continue to help it grow in acceptance.
Success in accomplishing an effective outcome is directly linked to an adherence of definable and replicable practice techniques. Wound selection, management of both endogenous and exogenous factors and appropriate epidermal grafting application with ongoing wound management are all final elements needed to complete this task.
As demonstrated, effective wound bed preparation enables even the most complicated wounds to close. Focus on having adequate blood flow, wound bed granulation, infection control and management of chronic inflammation satisfy a valuable link in the chain of success for wound closure with epidermal grafting.
References
1.Veves A, Sheehan P, Pham HT. A randomized, controlled trial of Promogran (a collagen/oxidized regenerated cellulose dressing) vs standard treatment in the management of diabetic foot ulcers. Arch Surg 2002; 137(7):822-7.
2.Game FL, Jeffcoate, WJ. Dressing and Diabetic Foot Ulcers: A Current Review of the Evidence. Plast Reconstr Surg. 2016 Sept; 138 (3Supp): 158S-64S.
3.Gottrup F, Cullen BM, Karlsmark T, Bischoff-Mikkelsen M, Nisbet L, Gibson MC. Randomized controlled trial on collagen/oxidized regenerated cellulose/silver treatment. Wound Repair Regen 2013; 21(2):216-25.
4.Braumann C, Guenther N, Menenakos C, Muenzberg H, Pirlich M, Lochs H, Mueller JM. Clinical experiences derived from implementation of an easy to use concept for treatment of wound healing by secondary intention and guidance in selection of appropriate dressings. Int Wound J 2011; 8(30):253-60.
5.Lobmann R, Ambrosch A, Schultz G, Waldmann K, Schiweck S, Lehnert H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia 2002; 45:1011-6.
6.Ulrich D, Smeets R, Unglaub F, Wöltje M, Pallua N. Effect of oxidized regenerated cellulose/collagen matrix on proteases in wound exudate of patients with diabetic foot ulcers. J Wound Ostomy Continence Nurs 2011;38(5):522-8.
REFERENCES
1.Mount Nittany Center for Wound Care, State College, Pa.
2.Schultz GS, Sibbald RG, Falanga V et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen. 2003;11(Supp 1):S1-S28
REFERENCES
1. Cowan, Linda, et al. Caution: when combining topical wound treatments, more is not always better. Wound Practice & Research: Journal of the Australian Wound Management Association 19.2 (2011): 60.
2. Armstrong D, Bates-Jensen B, Bohn G, et al. Expert recommendations for the use of hypochlorous solution: science and clinical application. Wounds. 2015.
3. Scanlon, E., and Stubbs, N. To use or not to use? The debate on the use of antiseptics in wound care. British journal of community nusring 7. Sup2 (2002): 8-20.
4. Atiyeh, B. S., Dibo, S. A., & Hayek, S. N. (2009). Wound cleansing, topical antiseptics and wound cleansing, topical antiseptics and wound healing. International Wound Journal, 6(6), 420-430.
5. Drosou A, Falabella A, Kirsner RS. Antiseptics on Wounds: An Area of Controversy. Wounds. 2003; 15(5):149-166.
6. Mulder GD, Cavorsi JP, Lee DK. Antimicrobial Agents in Wound Care. Wounds. 2007;19(7):173-182.
7. McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev.1999; 12(1):147-179.
8. Senior N. Some observations on the formulation and properties of chlorhexidine. J Soc Cosmet Chem. 24(4): 259-278.
9. Basrani BR, Manek S, Sodhi R, Fillery E, Manzur A. Interaction between sodium hypochlorite and chlorhexidine gluconate. J Endod. 2007; 33(8): 966-969.
10. Yasuda K, Ohmizo C, Katsu T. Potassium and tetraphenylphosphonium ion-selective electrodes for monitoring changes in the permeability of bacterial outer and cytoplasmic membranes. J Microbiol Methods. 2003;54(1):111-115.
11. Jovanovic A, Ermis R, Mewaldt RS, Shi L, Carson D. The Influence of Metal Salts, Surfactants, and Wound Care Products on Enzymatic Activity of Collagenase, the Wound Debriding Enzyme. Wounds. 24(9):242–253.
12. Safety and effectiveness of consumer antiseptics; topical antimicrobial drug products for over-the-counter human use; proposed amendment of the tentative final monograph; reopening of administrative record; proposed rule. Fed Regist 78:76444–76478. http://www.gpo.gov/fdsys/pkg/FR-2013-12-17/pdf/2013-29814.pdf
13. Khan M, Naqvi AH. Antiseptics, iodine, povidone iodine and traumatic wound cleansing. J Tissue Viability. 2006; 16(4):6-10.
14. Smith RG. Critical discussion of the use of antiseptics in acute traumatic wounds. J Am Podiatr Med Assoc. 2005; 95(2):148-153.
15. Schmitz G. Iodine oxidation by hydrogen peroxide and Bray–Liebhafsky oscillating reaction: effect of the temperature. Phys Chem Chem Phys. 13(15): 2011: 7102-7111. doi: 10.1039/C1CP00006C
16. Sakarya S, Gunay N, Karakulak M, Ozturk B, Ertugrul B. Hypochlorous Acid: an ideal wound care agent with powerful microbicidal, antibiofilm, and wound healing potency. Wounds. 2014; 26(12):342-350.
17. Thorn RM, Lee SW, Robinson GM, Greenman J, Reynolds DM. Electrochemically activated solutions: evidence for antimicrobial efficacy and applications in healthcare environments. Eur J Clin Microbiol Infect Dis. 2014; 31(5):641–653.
18. Edwards K. New Twist on an Old Favorite: Gentian Violet and Methylene Blue Antibacterial Foams. Adv Wound Care(New Rochelle). 2016; 5(1): 11–18.
19. Hydrofera Blue Classic Antibacterial Foam Dressing; SDS HB2214/HB4414-00 Safety [Online]; Hollister: Libertyville, IL, April 13, 2016. http://www.hollister.com/~/media/files/pdfs%E2%80%93for%E2%80%93download/sds/sds%E2%80%93hydrofera%E2%80%93blue%E2%80%93classic.pdf?la=en (accessed April 15, 2017)
20. Chao C, Runde D. Tap Water vs. Sterile Saline for Wound Irrigation. Am Fam Physician. 2015; 92(3). http://www.thennt.com/nnt/tap-water-for-wound-irrigation/. Accessed February 10, 2017
21. Kim P, Attinger C, Crist B, et al. Negative Pressure Wound Therapy with Instillation: Review of Evidence and Recommendations. Wounds. 2015; 27(12):S2-S19.
22. Wolvos T. Wound Instillation — The Next Step in Negative Pressure Wound Therapy. Lessons Learned from Initial Experiences. Ostomy Wound Manage. 2004; 50(11):56-66.