
Ralph J. Napolitano, Jr, DPM, CWSP, FACFAS is a double board-certified podiatrist and wound care specialist physician with OrthoNeuro, a multispecialty group based in central Ohio. He specializes in medicine, surgery and wound care of the foot, ankle and lower leg. He was the first podiatrist in the state of Ohio to earn the board certification Certified Wound Specialist Physician (CWSP). Areas of special clinical interest, include general wound care and healing, diabetic foot wounds, lower limb surgical salvage and management of surgical wound complications. Dr. Napolitano is a fellow of the American College of Foot and Ankle Surgeons and a diplomate of the American Board of Foot and Ankle Surgery and American Board of Wound Management. He holds clinical privileges at numerous area hospitals, surgery centers, and wound care centers. He is an Adjunct Clinical Assistant Professor in the Department of Specialty Medicine at Ohio University Heritage College of Osteopathic Medicine. He is also part of the clinical faculty for the Grant Medical Center Podiatric Medicine and Surgery Program. Dr. Napolitano was one of the physician founders that helped establish the Licking Memorial Hospital Wound Care Clinic over 15 years ago. He remains active publishing and lecturing locally and nationally on wound care and healing. In 2018, Dr. Napolitano was the only podiatrist in the world to have an article accepted into the Cureus Journal of Medical Science’s publishing competition on negative pressure wound therapy with instillation and dwell utilizing novel dressings. In 2019, during a limited commercial release, he was the first clinician in North America to trial DERMATACTM Drape, a new single-use, proprietary silicone-acrylic hybrid drape that is used with V.A.C.® Therapy. Dr. Napolitano is a consultant for 3M.
Napolitano_Current Dialogues in Wound Management_2020_Article_11
Modern negative pressure wound therapy (NPWT), now in its third decade of worldwide clinical use, is a well-documented treatment modality for the management of both acute and chronic wounds. NPWT systems promote wound healing by drawing wound edges together, removing infectious materials and exudate, and promoting the development of granulation tissue. Examples of wound type indications suitable for NPWT include decubitus wounds, diabetic foot ulcers, venous leg wounds, and surgical dehiscence wounds.1 This treatment modality was first promoted in 1989 by Chariker et al who described a suction drainage system for the treatment of incisional and cutaneous fistulae.2 The system they described used a gauze-filled dressing connected to a wall suction with therapeutic pressure set at 60-80 mmHg. Advances in NPWT technology saw the advent of polyurethane sponges in the 1990s which were favored for their positive effect on granulation tissue. Most of the devices in current use contain a similar open pore polyurethane dressing. Argenta and Morykwas pioneered the use of open pore polyurethane dressings coupled with sub atmospheric pressure at -125 mmHg.3 This configuration forms the basis of today’s contemporary NPWT device. Orgill and Bayer described four primary effects of NPWT on wound healing:4

It is well understood that to positively affect wound healing as described above, it is essential that NPWT systems establish a “seal” between the wound, dressing, and periwound skin. Traditionally, seal creation has been accomplished through the use of an adhesive, acrylic drape. Although very effective in seal creation and maintenance, it is not without some problems. Sensitivities to drape materials such as allergic dermatitis, periwound maceration, and pain upon removal exist for patients. Challenging dressing handling characteristics, such as sticking to gloves, and the inability to reposition the dressing once placed can be a hurdle for clinicians. Additionally, seal creation in certain anatomic regions can be difficult requiring “picture framing” or “windowpane” techniques in which additional strips of drape are needed to create an effective seal. Other shortcomings include increased cost related to the drape, glove wastage, and increased application time.
In 2019, KCI received clearance from the U.S. Food and Drug Administration for the DERMATAC™ Drape, a proprietary silicone-acrylic hybrid drape that provides operational benefits, as an accessory to V.A.C.® Therapy. Benefits of the DERMATAC™ Drape include:5-9

In my opinion, in addition to the above benefits, the DERMATAC™ Drape provides a seal without the need of accessory products around anatomically difficult areas (Figure 1).

Additional advantages of using the DERMATAC™ Drape for the patient include no significant discomfort during wear time (Figures 2-3).5-9
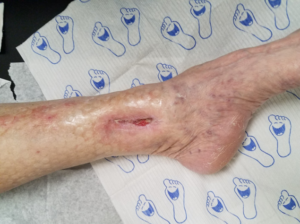
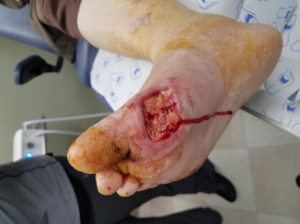
There are a few tips that should be considered when applying the DERMATAC™ Drape.
• Rare V.A.C.® Therapy track pad adherence issues have been reported with the DERMATAC™ Drape. This issue can be circumvented by increasing manual pressure when applying the track pad.
• Because of significantly improved handling properties, there is no need to “windowpane.”
• Care should be taken to apply the drape loosely, without overstretching, over the wound compared to traditional acrylic draping.
• A 5 cm drape border is necessary around the wound when using the DERMATAC™ Drape.
• As noted earlier, if drape repositioning is required, this can be done by carrying out a “lift and reposition” technique.
• If challenging anatomy is encountered, drape slits may be cut around curved areas and the DERMATAC™ Drape overlap carried out as necessary to remove the wrinkles.
Although the clinical benefits of V.A.C.® Therapy are beyond the scope of this brief article, this fairly new advance in the drape associated with V.A.C.® Therapy, the DERMATAC™ Drape, has already demonstrated clinical benefits for patients and clinicians.5-9
Patient data and photos courtesy of Ralph J. Napolitano, Jr, DPM, CWSP, FACFAS
As with any case study, the results and outcomes should not be interpreted as a guarantee or warranty of similar results. Individual results may vary depending on the patient’s circumstances and condition.
NOTE: Specific indications, contraindications, warnings, precautions, and safety information exist for these products and therapies. Please consult a clinician and product instructions for use prior to application. Rx only.
References
- Napolitano RJ Jr. Treatment of acute compartment syndrome sequela of the leg: a case report demonstrating negative pressure wound therapy with instillation and dwell utilizing a novel dressing and serial automated suction blister epidermal harvesting and grafting. Cureus. 2018;10(10):e3443. doi:10.7759/cureus.3443.
- Chariker ME, Jeter KF, Tintle TE, Bottsford JE. Effective management of incisional and cutaneous fistulae with closed suction wound drainage. Contemp Surg 1989;34:59-63.
- Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38(6):563-576.
- Orgill DP, Bayer LR. Negative pressure wound therapy: past, present and future. Int Wound J 2013;10(Suppl 1):15-19. doi:10.1111/iwj.12170.
- Napolitano RJ, Jr. Early use of a novel acrylic-silicone hybrid drape with negative pressure wound therapy in lower extremity wounds [abstract]. Presented at SAWC Fall 2019, October 12-14, 2019, Las Vegas, NV 2019.
- Kharkar P, Napolitano RJ, Jr., Lantis J et al. Assessment of a Novel Drape Containing Acrylic and Silicone-based Adhesives When Using Negative Pressure Wound Therapy [abstract]. Presented at SAWC Fall 2019, October 12-14, 2019, Las Vegas, NV 2019.
- Speyrer MS, Thibodeaux KT. Initial experience of negative pressure wound therapy using a novel hybrid adhesive drape [abstract]. Presented at the 2019 Wild on Wounds National Wound Conference, September 11-14, 2019, Las Vegas, NV 2019.
- Desvigne MN, Berman ML, Aragon J et al. Initial Experiences Applying Negative Pressure Wound Therapy With a Novel Drape Containing an Acrylic and Silicone-based Adhesive [abstract]. Presented at SAWC Fall 2019, October 12-16, 2019, Las Vegas, NV 2019.
- Galarza L. Initial clinical observations using a novel negative pressure wound therapy drape comprised of acrylic and silicone. Presented at SAWC Spring 2019, May 7-11, 2019, San Antonio, TX 2019.
©Copyright 2020 3M. All rights reserved. 3M and the other marks shown are marks and/or registered marks. Unauthorized use prohibited. PRA-PM-US-02507 (06/20).

