KCI, An Acelity Company. King Edward Court, King Edward Road, Knutsford, WA16 0BE, UK.2Faculty of Biology, Medicine and Health, University of Manchester, Manchester, M13 9PT, UK.
Dr Thomason is Director of Research Sciences at KCI, An Acelity Company and holds an honorary position at The University of Manchester where for over 15 years she has conducted her research on skin biology and wound healing. She has published multiple peer-reviewed manuscripts and book chapters focussing on understanding the cellular and molecular mechanisms of healing. Her research centres on developing clinically relevant models of delayed wound healing, including biofilm infection and using these models to understand how wound therapies can stimulate repair.
Thomason_Current Dialogues in Wound Management_2019_Volume 5_Issue 2
WOUND INFECTIONS AND BIOFILMS
Wound infection can cause a significant delay or prevent a wound from healing. Therefore, the appropriate management of infection is important to reduce healing times and the associated wound management costs. One of the main complications associated with wound infection is that the infection often manifests as a biofilm.1 Biofilms differ from free-floating, planktonic bacteria in that the bacteria form communities which adhere to a surface, such as the wound bed. Within these communities, bacteria secrete an extracellular polymeric substance (EPS) composed of polysaccharides, proteins, lipids and extracellular DNA. Bacteria within a biofilm are more tolerant to antimicrobial therapies, in part due to the slow growth rate of the bacteria within the biofilm and the protective nature of the EPS.2 Therefore, potent antimicrobials are required to effectively kill bacteria within a biofilm.
ANTIMICROBIAL ACTION OF SILVER
For centuries, silver has been known for its antimicrobial properties3 and exploited for medicinal use.4;5 It has a long standing history in the control wound infection, initially in solution or creams for the use in burns and more recently incorporated into wound dressings which deliver controlled and prolonged release of silver.6 Many different silver delivery systems have been used in wound care products, ranging from colloidal silver, silver sulfadiazine, silver proteins, silver salts, silver compounds, and nanocrystalline silver.
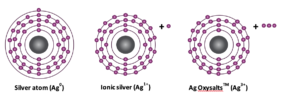
Metal silver (Ag0) is inert; it must be converted into its metallic state (Ag+) to exert antimicrobial action. In its metallic form, a silver atom contains a single electron in its outermost shell. When this electron is removed during a process called oxidation the silver becomes positively charged (AgIn its ionic form silver scavenges electrons from bacterial cells. In doing so it disrupts the bacterial cell wall, interferes with DNA replication and denatures proteins involved in bacterial metabolism, ultimately resulting in the bacterial death.
The efficacy of silver depends on a number of factors; the concentration of silver present, its solubility and the composition of the dressing/treatment itself. In addition, once silver is released from the dressing the wound environment can affect its efficacy; protein and chloride ions within wound fluid bind silver and thus reduce the concentration of bioavailability silver. Silver salts became popular in the 1960s when positively charged silver ions were complexed to negatively charged ions to produce electrically neutral compounds (AgCl, AgNO3, AgSO4), providing a more stable delivery system for silver. When exposed to wound fluid, these compounds break down to release ionic silver (Ag
Ag OXYSALTS™ Technology in wound care, silver-based therapies contain metallic silver (Ag0) or singly ionic silver (Ag1+). More recently a new silver technology, Ag OXYSALTS™ Technology (Ag7NO11) has been developed which can produce higher ionic states of silver (Ag1+, Ag2+ and Ag3+). Higher ionic states of silver are highly reactive and unstable, needing to fill their electron shells to gain stability.7 However, they can be stabilised with oxygen atoms to form Ag7NO11. Incorporation of this silver salt into wound dressings allows controlled delivery of higher ionic states of silver to the wound. When exposed to aqueous media, such as wound fluid, Ag OXYSALTS™ Technology break down to produce three ionic states of silver Ag1+, Ag2+ and Ag3+(Figure 1).8 The gaining of electrons by silver is known as reduction. The higher the reduction potential the stronger the affinity for electrons. The reduction potential for Ag1+ is 0.80 whereas for Ag2+ and Ag3+the reduction potential is 1.98 and 1.80, respectively. Thus, these higher ionic states of silver have a greater affinity for electrons.
Figure 1. Atoms are composed of positively charged nuclei surrounded by shells of negatively charged electrons (-). Metallic silver (Ag0) contains an equal number of positively charged protons within its nuclei and negatively charged electrons in the surrounding shells. When metallic silver loses an electron from its outer most shell it becomes positively charged, ionic silver (Ag1+). Ag OXYSALTS™ Technology lose up to 3 electrons forming higher ionic states of silver (Ag1+, Ag2+ and Ag)
AG OXYSALTS™ TECHNOLOGY – ANTIMICROBIAL AND ANTI-BIOFILM EFFICACY
Having a greater reduction potential or stronger affinity for electrons would indicate stronger antimicrobial action. In vitro studies have shown that a lower equimolar concentration of Ag OXYSALTS™ Technology is required to eradicate planktonic bacteria, prevent biofilm formation and eradicate established biofilms compare to Ag2O, AgO, Ag2SO4, AgNO3, silver sulfadiazine, and CuSO4.8Furthermore, Ag OXYSALTS™ Technology were shown to be superior to other silver compounds at reducing biofilm biomass.8 Subsequent studies have shown Ag OXYSALTS™ Technology to have superior antimicrobial and anti-biofilm efficacy against dual species biofilms, which are more difficult to eradicate, compared to AgNO3 and CuSO4.9 In addition, Ag OXYSALTS™ Technology have been shown to be effective against biofilms composed of multi-drug resistant bacteria.
SILVER CYTOTOXICITY
There has been a long-standing concern over the safety of silver therapies in wound care.11 The concerns are two-fold: firstly, the systemic absorption of silver and secondly local cytotoxic effects which may be detrimental to healing. The effects that silver dressings have on wound cells independent of infection are often overlooked, particularly as antimicrobials become more potent in attempt to combat biofilm infection. Many chronic wounds are stuck in the inflammatory phase of healing whereby heightened levels of inflammatory cells persist for prolonged periods of time within the wound. These inflammatory cells create tissue damage and a hostile wound environment that delays healing. Antimicrobials therefore need to be potent enough to combat wound infection, but not cause a cytotoxic effect on healing that may exacerbate an already hostile wound environment.
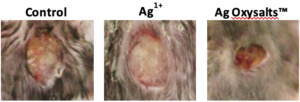
The effects of Ag OXYSALTS™ Technology on healing independent of infection has been examined. Ag OXYSALTS™ Technology had no effect on the closure of fibroblast scratch wounds. In contrast, Ag OXYSALTS™ Technology promoted the healing of keratinocyte scratch wounds.12 The ability of Ag OXYSALTS™ Technology to promote healing independent of infection was also confirmed in a murine wound model, whereby wounds treated with Ag OXYSALTS™ Technology dressings were significantly smaller, with greater re-epithelialisation and reduced inflammation compared to control treated wounds (Figure 2). How Ag OXYSALTS™ Technology promote healing independent of infection has been investigated. Ag OXYSALTS™ Technology were found to be the only silver compound to release oxygen during their breakdown.12 The release of oxygen may be sufficient to shift wounds out of a hypoxic state or provide additional oxygen to the wound to support cell migration. In addition, dressings containing Ag OXYSALTS™ Technology were able to catalyse the breakdown of hydrogen peroxide to oxygen and water.12 Hydrogen peroxide plays a vital role in the early stages of wound healing;13 however, in chronic wounds high numbers of inflammatory cells which release hydrogen peroxide as a mechanism of killing bacteria contribute to damaging levels within the in the wound. Ag OXYSALTS™ Technology may therefore contribute to reducing detrimental levels of hydrogen peroxide within a wound and in doing so release oxygen during this process.
SILVER TOLERANCE AND RESISTANCE
The increased use of silver in wound care has raised concerns over bacteria developing tolerance or resistance to silver therapies. Resistance is defined as the inherited ability of bacteria to grow in high silver concentrations
Figure 2. Ag OXYSALTS™ Technology promote healing independent of infection. Uninfected excisional mouse wounds treated for 3 days with control or silver dressings. In contrast to wounds treated with silver chloride dressings (Ag1+), wounds treated with Ag OXYSALTS™ Technology (Ag3+) show accelerated healing compared to control treated wounds.
irrespective of the duration of treatment.14 In contrast, tolerance is the temporary ability of bacterial to survive high silver concentrations which would normally be lethal. Tolerance may or may not be inherited; however, unlike resistance tolerance in is often achieved by slowing down bacterial growth and reducing metabolism. Therefore, a longer exposure to silver, rather than a higher concentration, is required to kill tolerant bacteria compare to susceptible bacteria.14 The molecular basis of silver resistance is well understood. Bacteria display resistance through the expression of silver resistance genes; however, many studies have shown limited presence of these resistance genes in clinical isolates.15;16Furthermore, although bacteria may harbour silver resistant genes, in many cases they do not express the genes to a significant level and therefore display little or no resistance to low levels of silver.17 The likelihood of developing silver resistance is reduced if the silver has rapid and sustained antimicrobial activity.18Studies have found that dressings containing Ag OXYSALTS™ Technology were effective against bacteria expressing silver resistant genes when other silver dressings were ineffective.
SUMMARY
In wound care, a wide variety of silver formulations have been used to treat wound infections. There has been a recent increase in the use of wound dressings incorporating silver compounds; however, clinical concerns still remain over their ability to combat biofilm infections, and whether they cause local cytotoxicity or adversely affect healing independent of infection. Dressings containing Ag OXYSALTS™ Technology are the only commercially available silver dressings which produce silver at higher oxidative states (Ag2+and Ag3+). Despite being potently antimicrobial and effectively killing bacteria within a biofilm, Ag OXYSALTS™ Technology do not have an adverse effect on healing; on the contrary, in vivo studies have shown Ag OXYSALTS™ Technology to promote healing independent of infection.
References
1.Schultz G, Bjarnsholt T, James GA et al. Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair Regen 2017;25(5):744-757. doi:10.1111/wrr.12590.
2.Snyder RJ, Bohn G, Hanft J et al. Wound Biofilm: Current Perspectives and Strategies on Biofilm Disruption and Treatments. Wounds 2017;29(6):S1-S17.
3.Russell AD, Hugo WB. Antimicrobial activity and action of silver. Prog Med Chem 1994;31:351-370.
4.Fan FF, Bard AJ. Chemical, Electrochemical, Gravimetric, and Microscopic Studies on Antimicrobial Silver Films. Journal of Physical Chemistry B 2002;106(2):279-287.
5.Klasen HJ. Historical review of the use of silver in the treatment of burns. I. Early uses. Burns 2000;26(2):117-130.
6.Klasen HJ. A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. Burns 2000;26(2):131-138.
7.Djokic SS. Deposition of silver oxysalts and their antimicrobial properties. Journal of the Electrochemical Society, 2004;151(6):C359-C364.
8.Lemire JA, Kalan L, Bradu A, Turner RJ. Silver oxynitrate, an unexplored silver compound with antimicrobial and antibiofilm activity. Antimicrob Agents Chemother 2015;59(7):4031-403
9. doi:10.1128/AAC.05177-14.9.Lemire JA, Kalan L, Gugala N, Bradu A, Turner RJ. Silver oxynitrate – an efficacious compound for the prevention and eradication of dual-species biofilms. Biofouling 2017;33(6):460-469. doi:10.1080/08927014.2017.1322586.
10.Kalan LR, Pepin DM, Ul-Haq I, Miller SB, Hay ME, Precht RJ. Targeting biofilms of multidrug-resistant bacteria with silver oxynitrate. Int J Antimicrob Agents 2017;49(6):719-726. doi:10.1016/j.ijantimicag.2017.01.019.
11.Nagoba BS, Suryawanshi NM, Selkar SP. Cytotoxicity of silver dressings-time to rethink and react. Int Wound J 2013;10(5):616. doi:10.1111/iwj.12139.
12.Thomason HA, Lovett JM, Spina CJ, Stephenson C, McBain AJ, Hardman MJ. Silver oxysalts promote cutaneous wound healing independent of infection. Wound Repair Regen 2018. doi:10.1111/wrr.12627.
13.Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 2009;459(7249):996-999. doi:10.1038/nature08119.
14.Brauner A, Fridman O, Gefen O, Balaban NQ. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nature Reviews Microbiology 2016;14(5):320-330. doi:10.1038/nrmicro.2016.34.
15.Percival SL, Woods E, Nutekpor M, Bowler P, Radford A, Cochrane C. Prevalence of silver resistance in bacteria isolated from diabetic foot ulcers and efficacy of silver-containing wound dressings. Ostomy Wound Manage 2008;54(3):30-40.
16.Woods EJ, Cochrane CA, Percival SL. Prevalence of silver resistance genes in bacteria isolated from human and horse wounds. Vet Microbiol 2009;138(3-4):325-329. doi:10.1016/j.vetmic.2009.03.023.
17.Loh JV, Percival SL, Woods EJ, Williams NJ, Cochrane CA. Silver resistance in MRSA isolated from wound and nasal sources in humans and animals. Int Wound J 2009;6(1):32-38. doi:10.1111/j.1742-481X.2008.00563.x.
18.Chopra I. The increasing use of silver-based products as antimicrobial agents: a useful development or a cause for concern? J Antimicrob Chemother 2007;59(4):587-590. doi:10.1093/jac/dkm006.
19.Finley PJ, Norton R, Austin C, Mitchell A, Zank S, Durham P. Unprecedented Silver Resistance in Clinically Isolated Enterobacteriaceae: Major Implications for Burn and Wound Management. Antimicrob Agents Chemother 2015;59(8):4734-4741. doi:10.1128/AAC.00026-15.