
Dr. Subhas Gupta is the Chairman of the Department of Plastic Surgery at Loma Linda University in Southern California where he holds the academic rank of Professor and is the director of research. He is an innovative plastic surgeon who holds a PhD in Medical Informatics focused on the introduction of new technologies to clinical care. Dr. Gupta is the director of the Loma Linda University Soft Tissue Reconstruction Team and directs a bio-investigative cell biology and wound healing laboratory. Dr. Gupta is a consultant for 3M.
Gupta_Current Dialogues in Wound Management_2020_Article_19
As surgery has advanced from simply removing the effects of ailments to addressing the cause of morbidity, incisions grew in length and complexity. While minimally invasive and robotic-assisted surgeries have reversed this trend, the increasingly complex physiology of patients who are older, more immunosuppressed, and have an increasing number of wound impairing risk factors has made the goal of optimizing the healing of surgical incisions paramount in the care of these patients.
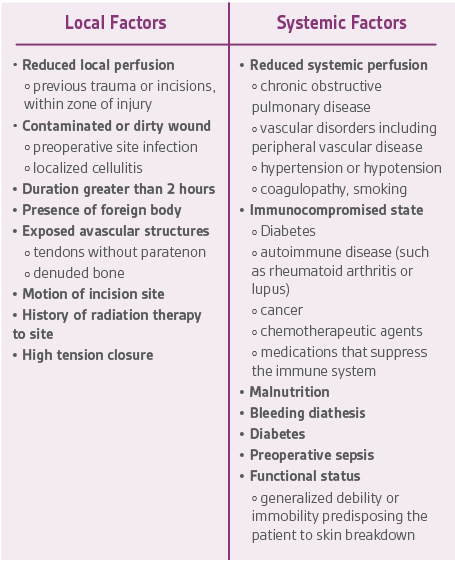
Incisional wound healing is influenced by a number of factors. The complexity and anatomic location of surgery both have an impact on wound healing, with increasing risk determined by factors including tissue trauma, contamination, motion at the surgical site, and wound closure tension.1 Cardiothoracic, abdominal, and lower extremity vascular orthopedic incisions are at the highest risk for complications.2 Patient risk factors and comorbidities also present risk for impaired surgical site healing. A typical high-risk incision for healing compromise is seen in Figure 1. Local and systemic factors that may impair post-operative incision healing are summarized in Table 1.
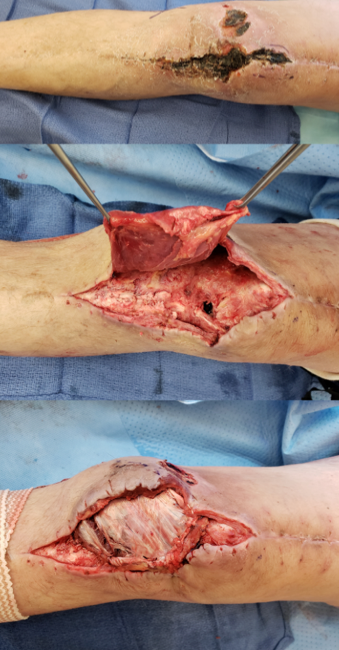
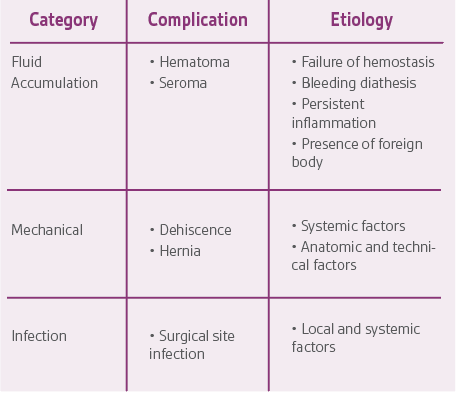
Specific complications of surgical incisions are listed in Table 2 along with their presumptive etiologic factors. In addition to preoperative patient selection, screening, and optimization, there are intraoperative and postoperative maneuvers to optimize healing and minimize complications.
INTRAOPERATIVE MANEUVERS TO OPTIMIZE INCISION HEALING
The intraoperative phase is the period of time from when the patient arrives on the operating table to when the wound is closed.3 There are local and systemic factors to optimize along with operating theater environmental factors. There are many factors from Table 1 that cannot be optimized, but an evidence-based approach to address as many as possible is the ideal strategy to enhance surgical incision healing.
Systemic Factors
MRSA and Perioperative Antibiotics
First, it is important to screen for a history of methicillin-resistant Staphylococcus aureus (MRSA), as patients who carry this are more likely to develop S. aureus surgical site infections (SSIs).4 If the patient is positive for MRSA, apply mupirocin intranasally twice daily and bathe in chlorohexidine-gluconate (CHG) daily for up to 5 days immediately before the operation.5,6 Additionally, administering a single dose of vancomycin, 15 mg/kg up to a maximum of 2 g/dose, 120 minutes before the procedure may be beneficial for MRSA carriers.5-7 For all patients, however, regardless of the MRSA screening results, administer cefazolin or cefuroxime as prophylaxis 60 minutes prior to the surgical incision, with a redosing plan for procedures lasting 4 hours or longer.8 In addition, discontinue prophylactic antibiotics within 24 hours of the completion of surgery as postoperative continuation of antibiotics has neither benefit nor harm in reducing SSI rates when compared to a single dose of antibiotic prophylaxis.9,10
Physiologic Optimization
Once the procedure has begun, special attention should be paid to maintaining normothermia, glycemic control, and enhancing oxygenation throughout the procedure. Warming blankets can be used as an active measure in maintaining intraoperative normothermia. During surgery, glycemic control should also be implemented, targeting glucose levels to be less than 200 mg/dL since elevated hemoglobin A1C levels are associated with higher risk of SSIs.11,12 Enhanced perioperative oxygenation also lowers the prevalence of SSIs as well.13
Local Factors
Prepping the surgical site should be done comprehensively and with organization. Chlorhexidine with or without isopropyl alcohol has been shown to achieve enhanced skin antisepsis compared to other preparations.14 Careful debridement of all non-viable tissue is another best practice to reduce incisional healing challenges.
Once the surgical procedure is complete, but before closing any layer of the incision, the wound should be irrigated with a physiologic solution and/or an antiseptic solution such as PVP-Iodine or chlorhexidine. Diluted chlorhexidine 0.05% has been demonstrated to work on contact without needing the wound to dry.15 When closing the superficial layers of the incision, consider using antimicrobial sutures, especially in contaminated cases. In addition, the use of silver dressings to seal the incision from exogenous contaminants reduces bacterial infection and bioburden.16 Skin glues provide a strong antimicrobial barrier to the incision site, but allergic reactions are not uncommon.17
Environmental Factors
Hygiene of the operating team’s hands plays an undeniable role in ensuring the safety of the patients. S. aureus can survive on hands for at least 150 minutes. Therefore, it is crucial for the staff to wash their hands throughout the day to minimize the risk of infection. The most effective method is using 60-80% ethanol to scrub the hands, as it is found to be effective within 30 seconds without harming, dehydrating, or causing irritation of the skin.18 Once the hands are disinfected, double gloving of the staff with indicator system and changing outer gloves every 60 mins is shown to help identify perforations in the timeliest manner, preventing blood-borne infections. In fact, the risk of contamination from blood is 13 times higher when single gloving compared to double gloving.19 Furthermore, research shows that double gloving with an indicator system provides even better protection.19,20
Once the preparation of the patient and staff is finished and the procedure is ready to begin, operating room (OR) traffic, identified by the number of door openings during the procedure, must be limited as much as possible. Opening the door too many times or keeping the door open for too long overwhelms the positive laminar flow systems that are designed to prevent potentially contaminated air from flowing in. Furthermore, the number of people in the OR, linked to the number of door openings, is known to be one of the most important factors related to the bacterial count in the OR. Therefore, OR traffic is a safety concern, as it compromises OR sterility and potentially increases infection rates.21
POSTOPERATIVE MANEUVERS TO OPTIMIZE INCISION HEALING
In the immediate postoperative period, protection against bacterial invasion is a key measure in optimizing healing.22 The most recent Centers for Disease Control and Prevention (CDC) guidelines (2017), regarding preventing surgical site infections, reaffirm the recommendation that primarily closed incisions should be covered with a sterile dressing for 24-48 hours postoperatively. Common postoperative dressings for surgical incisions include non-adherent dressings with non-woven adhesive tape and antibacterial gauze dressings. If the dressing must be changed prior to 48 hours, the dressing should be changed using sterile-technique.23
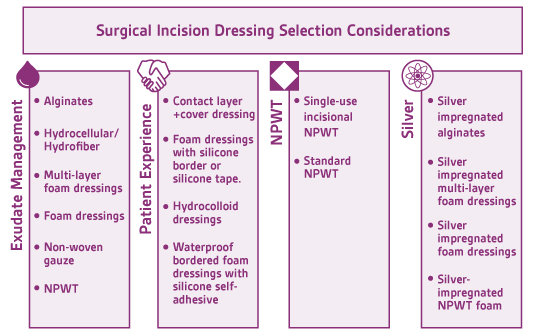
Although there is general consensus among wound care clinicians regarding occlusive sterile dressing application post-operatively, there is insufficient data in the literature supporting the use of one dressing over another.22,24 There are a multitude of published studies aimed at evaluating standard post-operative dressings but most are small-scale, and may be rife with other methodological limitations including possible confounding factors.24 Wound dressings may be selected for the postoperative period in the context of managing wound exudate, patient experience, wound location, and/or patient risk factors for complicated wound healing and infection.
Exudate Management
Exudate management is a key principle in evidence-based wound care. Excessive or insufficient exudate may cause delayed wound healing.24 The wound care clinician must utilize a dressing that has the appropriate exudate absorption capacity and the dressing must be absorptive enough to manage the amount of wound exudate and also accommodate a reasonable dressing change frequency. This is also extremely important in the postoperative period to reduce the number of sterile dressing changes done in the first 48 hours and/or prior to epithelial bridging.
There are multiple standard dressing options that provide exudate management and decrease the chance of bacterial compromise by acting as a physical barrier. Alginates and hydrocellular/hydrofiber dressings are most appropriate for wounds with moderate to high levels of exudate as they absorb drainage and become a moist dressing that is in direct contact with the wound bed. Bordered and non-bordered foams also provide excellent exudate management. Foam dressings are versatile and may be used as a primary dressing or in conjunction with an alginate, hydrocellular, or hydrofiber dressing. Dry gauze may also be utilized for exudate management in wounds with small-to-moderate levels of exudate. As dry gauze does not have the same absorptive properties as the previously mentioned alternatives, dry gauze must be changed more frequently. Excessive exudate may also cause periwound skin maceration, if not managed appropriately. Barrier films may be applied to periwound skin for protection against moisture, especially if dressings are to be changed less frequently.
Patient Experience
Periwound skin breakdown represents a risk factor for surgical site infection, due to local inflammation and loss of skin integrity, and a risk for patient dissatisfaction due to pain, itching, and additional wounds. Periwound skin breakdown in surgical incisions may occur from moisture exposure (moisture-associated skin damage), adhesive-related skin injury from adhesive skin stripping or skin tears, tension from high-adherent adhesives causing blisters, or allergic or contact dermatitis from adhesives. Changing dressings to a lower-adherent adhesive, such as soft silicone, or to a more absorbent dressing that locks moisture away from skin (e.g. multilayer foam, hydrofiber, alginate) may improve periwound skin outcomes and improve patient tolerance of the wound care dressing and wound care.25 Flexible post-operative foam dressings that are soft-silicone and self-adhesive may offer a greater range of motion while minimizing periwound skin breakdown from tension and moisture exposure, and improve pain control with dressing changes over traditional high-adhesive and less flexible post-operative dressings.26,27
Silver
Topical antimicrobials, such as silver, have become increasingly used as an adjunct to systemic antibiotics for prevention of infection.28 Although the antimicrobial properties of silver have been acknowledged for centuries, several systematic reviews have shown conflicting evidence on the antimicrobial properties of silver in wound care dressings. Davies et al attribute this to the varying amount, type, content, and compound of silver in dressings on the market.28
A systematic review by the CDC found that there are uncertain trade-offs when applying antimicrobial dressings to primarily closed surgical incisions for the prevention of SSI. The CDC has not made a current recommendation on antimicrobial dressings applied to primarily closed incisions for prevention of SSI.23 Further research is needed to recommend use.
NPWT
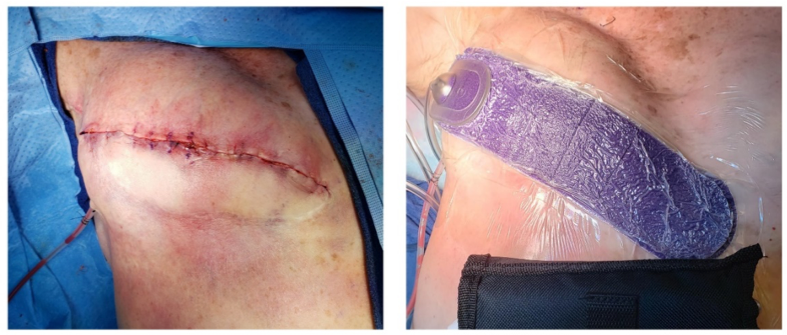
Postoperative negative-pressure wound therapy (pNPWT), either standard or simple, single-use battery-powered NPWT devices, may decrease risk for wound dehiscence, hematoma, and seroma.29 When compared to standard post-operative dressings, pNPWT may improve wound healing in heavily draining wounds, wounds with significant soft tissue damage, and wounds closed under tension.30 Scalise and colleagues found that pNPWT may be of particular benefit in preventing post-surgical wound complications, including surgical site infection and wound dehiscence, in high-risk surgical oncology patients, and in abdominal surgeries in obese and morbidly obese patients.30 The World Health Organization (WHO) 2016 guidelines recommend the use of pNPWT on primarily closed incisions at risk for comprised wound healing to prevent SSI. It should be noted that pNPWT may not be the most appropriate surgical dressing for those with limited access to resources.31 Figure 3 is an example of pNPWT placement over a high-risk breast reconstruction incision.
CONCLUSION
The evidence strongly suggests that the techniques discussed above are effective in reducing the incidence of SSIs. It is even possible that a synergistic effect occurs when these multiple interventions are combined; however, compliance and implementation remain a challenge. Adherence with the bundles may be a key to success in reducing SSIs, but there is great difficulty in auditing compliance with the total bundle as well as compliance with each of the individual components.32
Consideration for the rising costs of healthcare, institutional wound care formulary, the multiple factors influencing wound healing, and the risks for delayed or complex wound healing in patients, guide dressing selection for post-operative wound care. Providers are supported by a general knowledge of risks to incision healing and the potential use and benefits of various post-operative incisional dressings.
References
- Gupta SC. A practical evolution of the reconstructive ladder across care settings: developing a value-based reconstructive pathway. Current Dialogues in Wound Management 2019;5(3):5-7.
- Willy C, Agarwal A, Andersen CA, et al. Closed incision negative pressure therapy: international multidisciplinary consensus recommendations. Int Wound J. 2017;14:385-398.
- Joint Commission International. Evidence-based principles and practices for preventing surgical site infections. Oakbrook Terrace, IL: The Joint Commission; 2018.
- Rao N, Cannella BA, Crossett LS, Yates AJ, Jr., McGough RL, III, Hamilton CW. Preoperative screening/decolonization for Staphylococcus aureus to prevent orthopedic surgical site infection: prospective cohort study with 2-year follow-up. J Arthroplasty 2011;26(8):1501-1507. doi:10.1016/j.arth.2011.03.014.
- Schweizer ML, Chiang HY, Septimus E et al. Association of a bundled intervention with surgical site infections among patients undergoing cardiac, hip, or knee surgery. J Am Med Assoc 2015;313(21):2162-2171. doi:10.1001/jama.2015.5387.
- Bode LG, Kluytmans JA, Wertheim HF et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med 2010;362(1):9-17. doi:10.1056/NEJMoa0808939.
- Dunivan G, Krantz T. Putting surgical site infection bundles into practice. www contemporaryobgyn net. 2018; https://www.contemporaryobgyn.net/view/putting-surgical-site-infection-bundles-practice. Accessed July 31, 2020.
- Rozario D. Can surgical site infections be reduced with the adoption of a bundle of simultaneous initiatives? The use of NSQIP incidence data to follow multiple quality improvement interventions. Can J Surg 2018;61(1):68-70.
- Salkind AR, Rao KC. Antiobiotic prophylaxis to prevent surgical site infections. Am Fam Physician 2011;83(5):585-590.
- World Health Organization. Global Guidelines for the Prevention of Surgical Site Infection. Web Appendix 25: Summary of systematic review on surgical antibiotic prophylaxis prolongation. Geneva, Switzerland: World Health Organization; 2018.
- Berrios-Torres SI, Umscheid CA, Bratzler DW et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surgery 2017;152(8):784-791. doi:10.1001/jamasurg.2017.0904.
- Shohat N, Muhsen K, Gilat R, Rondon AJ, Chen AF, Parvizi J. Inadequate Glycemic Control Is Associated With Increased Surgical Site Infection in Total Joint Arthroplasty: A Systematic Review and Meta-Analysis. J Arthroplasty 2018;33(7):2312-2321. doi:10.1016/j.arth.2018.02.020.
- World Health Organization. Global Guidelines for the Prevention of Surgical Site Infection. Web Appendix 13a: Summary of the systematic review on perioperative oxygenation, in 2016 (Superseded by 13b and 13c). Geneva, Switzerland: World Health Organization; 2018.
- Adams D, Quayum M, Worthington T, Lambert P, Elliott T. Evaluation of a 2% chlorhexidine gluconate in 70% isopropyl alcohol skin disinfectant. J Hosp Infect 2005;61(4):287-290. doi:10.1016/j.jhin.2005.05.015.
- Willy C, Scheuermann-Poley C, Stichling M, von Stein T, Kramer A. [Importance of wound irrigation solutions and fluids with antiseptic effects in therapy and prophylaxis : Update 2017]. Unfallchirurg 2017;120(7):549-560. doi:10.1007/s00113-017-0375-5.
- Siah CJ, Yatim J. Efficiacy of a total occlusive ionic silver-containing dressing combination in decreasing risk of surgical site infection: an RCT. J Wound Care. 2011;20:561-568.
- Nakagawa S, Uda H, Sarukawa S, Sunaga A, Asahi R, Chi D, Yoshimura K. Contact dermatitis caused by dermabond advanced use. Plast Reconstruct Surg Global Open. 2018;6:e1841.
- Kampf G, Kramer A. Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clin Microbiol Rev 2004;17(4):863-93, table of contents. doi:10.1128/CMR.17.4.863-893.2004.
- Laine T, Aarnio P. Glove perforation in orthopaedic and trauma surgery. A comparison between single, double indicator gloving and double gloving with two regular gloves. Journal of Bone and Joint Surgery British Volume 2004;86(6):898-900. doi:10.1302/0301-620x.86b6.14821.
- Florman S, Burgdorf M, Finigan K, Slakey D, Hewitt R, Nichols RL. Efficacy of double gloving with an intrinsic indicator system. Surgical Infections 2005;6(4):385-395. doi:10.1089/sur.2005.6.385.
- Mears SC, Blanding R, Belkoff SM. Door Opening Affects Operating Room Pressure During Joint Arthroplasty. Orthopedics 2015;38(11):e991-e994. doi:10.3928/01477447-20151020-07.
- Wound Ostomy and Continence Nurses Society, Doughty DB, McNichol LL. Wound, Ostomy and Continence Nurses Society Core Curriculum: Wound Management. Philadelphia, PA: Wolters Kluwer; 2016.
- Rogers SO, Jr. Surgical Perspective: Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection 2017. Surgical Infections 2017;18(4):383-384. doi:10.1089/sur.2017.097.
- Dumville JC, Gray TA, Walter CJ, Sharp CA, Page T. Dressings for the prevention of surgical site infection. Cochrane Database of Systematic Reviews 2014;(9):CD003091. doi:10.1002/14651858.CD003091.pub3.
- Wongkietkachorn A, Surakunprapha P, Titapun A, Wongkietkachorn N, Wongkietkachorn S. Periwound Challenges Improve Patient Satisfaction in Wound Care. Plastic and Reconstructive Surgery Global Open 2019;7(3):e2134. doi:10.1097/GOX.0000000000002134.
- Beele H, Van Overschelde P, Olivecrona C, Smet S. A prospective randomized controlled clinical investigation comparing two post-operative wound dressings used after elective hip and knee replacement; Mepilex Border Post-Op versus Aquacel surgical. International Journal of Orthopaedic and Trauma Nursing 2020;38:100772. doi:10.1016/j.ijotn.2020.100772.
- Chowdhry M, Chen AF. Wound dressings for primary and revision total joint arthroplasty. Annals of Translational Medicine 2015;3(18):268. doi:10.3978/j.issn.2305-5839.2015.09.25.
- Davies P, McCarty S, Hamberg K. Silver-containing foam dressings with Safetac: a review of the scientific and clinical data. J Wound Care 2017;26(Sup6a):S1-S32. doi:10.12968/jowc.2017.26.Sup6a.S1 rtyp- generic.
- Hyldig N, Birke-Sorensen H, Kruse M et al. Meta-analysis of negative-pressure wound therapy for closed surgical incisions. Br J Surg 2016;103(5):477-486. doi:10.1002/bjs.10084.
- Scalise A, Calamita R, Tartaglione C et al. Improving wound healing and preventing surgical site complications of closed surgical incisions: a possible role of Incisional Negative Pressure Wound Therapy. A systematic review of the literature. Int Wound J 2016;13(6):1260-1281. doi:10.1111/iwj.12492.
- Allegranzi B, Zayed B, Bischoff P et al. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infectious Diseases 2016;16(12):e288-e303. doi:10.1016/S1473-3099(16)30402-9.
- Schriefer J, Hilt S, Sanders J et al. Implementation of a Pediatric Orthopaedic Bundle to Reduce Surgical Site Infections. Orthopedic Nursing 2017;36(1):49-59. doi:10.1097/NOR.0000000000000312.
NOTE: Specific indications, limitations, contraindications, warnings, precautions and safety information exist for these products and therapies. Please consult a clinician and product instructions for use prior to application. Rx only.
© 2020 3M. All rights reserved. 3M and the other marks shown are marks and/or registered marks. Unauthorized use prohibited. PRA-PM-US-02692 (09/20)

