
Ms. Weir has been Board Certified by the Wound, Ostomy and Continence Nurses Certification Board since 1985 (CWON) and American Board of Wound Management since 2004 (CWS). Dot is the co-chair of the Symposium on Advanced Wound Care, and was on the founding Board of the Association for the Advancement of Wound Care. Dot is a frequent lecturer on all aspects of wound management, and has authored and co-authored many journal articles and 9 book chapters.
Weir_Current Dialogues in Wound Management_2018_Volume 4_Issue 3
NOTE: As with any case study, the results and outcomes should not be interpreted as a guarantee or warranty of similar results. Individual results may vary depending on the patient’s circumstances and condition.
Acute wound healing has been extensively studied and taught for decades with the understanding that the acute wound progresses through the series of overlapping phases of hemostasis, inflammation, proliferation (including granulation, angiogenesis, and epithelialization), and ultimately remodeling.1Chronic wounds have been described as those that either fail to progress through the wound healing phases and become stalled at some point. Usually inflammation or something occurs during proliferation that alters the healing environment and re-ignites the inflammatory process.
An expected outcome of acute healing is a fully epithelialized closed wound. Partial thicknesswounds heal by epithelial cell migration over the wound bed and return to the original structure and function. Full thickness wounds heal by angiogenesis and the formation of granulation tissue, epithelialization and, ultimately, remodeling with formation of a scar. The resulting tissue is functional but does not contain the original tissue that was lost in the injury.
Practitioners in wound care are charged with the evaluation of the wound and the determination of what has caused or may cause the wound to deviate from the expected events of healing. A patient may present with a wound acquired in a matter of days, but it may be an acute wound on a chronic patient. Multiple factors including co-existing comorbid conditions (such as chronic venous insufficiency, diabetes), or arterial disease requiring specific supportive management (such as compression and offloading), drug therapy (such as steroids, anti-TNF drugs for rheumatoid arthritis and other autoimmune disorders and chemotherapeutic agents), or patient-controlled challenges (such as smoking and inadequately controlled blood glucose) contribute to altered wound healing in chronic patients (Figure 1). Commonly, though, a percentage of wounds become chronic with time in spite of evidence-based care. Our attention must then be directed at the potential barrier(s) to healing that must be addressed in order to change the wound’s trajectory.
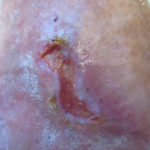 Figure 1. A seemingly benign wound present for greater than 20 years.
Figure 1. A seemingly benign wound present for greater than 20 years.
For almost two decades, the concept of wound bed preparation (WBP) has been well researched, discussed and documented. In 2003, a group of practitioners and scientists met to address the key barriers to healing to determine and provide key clinical concepts for managing most chronic wounds, and the same concepts are used by clinicians in the management of open acute wounds.5 The result of that meeting was the development of the acronym TIME (Tissue, Infection/Inflammation, Moisture and Edge) , to provide a structured approach to management. Today, the concept of TIME continues to be a sensible approach to address and correct the non-disease related barriers to healing.
APPROACHING WOUND BED PREPARATION
The aim of WBP is to move the wound into a state of readiness to heal. Doing so facilitates endogenous healing, as well as enhances the effectiveness of other therapeutic measures by integrating proven concepts to build a platform for treatment. Using a systematic approach such as the TIME concept allows us to organize medical procedures into a holistic approach that can be used to evaluate and remove barriers to the wound healing process.
T = TISSUE
The barriers to healing that must be addressed with WBP are interrelated from a cause and effect standpoint. From a macroscopic standpoint, the evidence of the most prevalent barrier to healing is necrotic tissue, visually evident proteinaceous debris, or coagulum which will stimulate the presence of bacteria and ultimately the secretion of proteases, protein-degrading enzymes produced in response to inflammation and bacterial presence, creating a self-perpetuating hostile wound environment. Removal of devitalized or necrotic tissue must occur in appropriate wounds. The importance of frequent debridement has been shown in large retrospective analyses of large clinical trials to be impactful in improving wound healing in diabetic foot ulcers and venous leg ulcers.7It must be stated that before launching into a debridement process in the presence of intact, dry eschar, one must be certain of the vascular status and long-term goals of the patient. The presence of a dry eschar on the limb of a patient with insufficient vascular perfusion is likely going to be left intact so as not to set the patient up for the potential site for bacterial growth. The presence of a dry eschar in a terminally ill patient would be easily and relatively painlessly managed with a dry dressing versus initiation of debridement, potentially causing pain and more frequent dressing changes.
The choice of the methodology used for removal of devitalized tissue will be driven by
•the patient’s comorbid conditions (medications, diabetes, chronic venous insufficiency, etc.),
•the wound type,
•the urgency of the need,
•the setting of care, and
•skill set of the practitioner.
TYPES OF DEBRIDEMENT
SURGICAL – The patient is taken to the operating room and deeply debrided under anesthesia.
SHARP – A sharp instrument (scalpel, curette, scissors/forceps) is used to remove devitalized tissue (Figures 2 & 3).
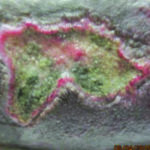 Figure 2. Venous leg ulcer pre-debridement with a curette.
Figure 2. Venous leg ulcer pre-debridement with a curette.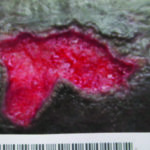 Figure 3. Venous leg ulcer post-debridement with a curette.
Figure 3. Venous leg ulcer post-debridement with a curette.
ENZYMATIC: The use of topically applied enzyme to cleave the bonds of devitalized from healthy tissue. In the US, this is a prescription drug, clostridial collagenase, which has the ability to cleave devitalized tissue in seven areas to more rapidly separate and remove necrotic tissue. It must be used in a moist environment, applied (dosed) in a 2-mm thick coating over the entire wound surface and applied daily (see Figures 4 and 5).
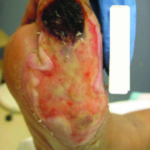 Figure 4. Diabetic foot wound prior to enzymatic debridement
Figure 4. Diabetic foot wound prior to enzymatic debridement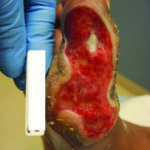 Figure 5. Same diabetic foot wound after use of enzymatic debridement
Figure 5. Same diabetic foot wound after use of enzymatic debridement
MECHANICAL – The use of a mechanical means to remove tissue including abrading (scrubbing with gauze) or other means such as monofilament pads which have been found to be more effective and less painful. Ultrasound and hydrotherapy are also forms of mechanical debridement (see Figure 6).
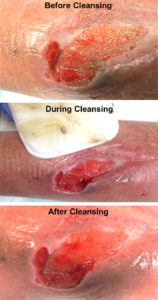 Figure 6. Wound before and after mechanical cleansing with monofilament pad
Figure 6. Wound before and after mechanical cleansing with monofilament pad
AUTOLYTIC: The liquefaction of surface debris utilizing endogenous naturally occurring proteases in wound exudate to lyse and liquify necrotic debris. It may be slower and dependent upon an individual’s immune system.
SURFACTANT AUTOLYTIC: The use of a concentrated surfactant presented in a gel delivery system which provides local microscopic cleansing and separation of debris that is not firmly attached to the wound surface. The micelles present in the surfactant gel softens, loosens, and traps debris and necrotic tissue (see Figures 7 & 8).
Implication of TISSUE: Properly prepared tissue will begin to granulate and be conducive and receptive to migration of epithelial cells over the surface for wound closure.
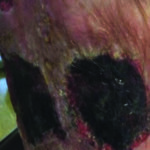 Figure 7. Ulcers prior to cleansing with surfactant autolytic debridement
Figure 7. Ulcers prior to cleansing with surfactant autolytic debridement
I = INFECTION AND/OR INFLAMMATION
Infection versus inflammation has been covered elsewhere in this journal issue; from the standpoint of WBP, it is part of the evaluation of the wound bed and an impetus for the production of proteases and the evolution of a hostile wound environment. In approaching a wound from the standpoint of preparation for healing, managing the presence of bacteria, not only as a nidus for infection, but also as a stimulus for protease production, is critical. There can be a vicious cycle of bacterial presence leading to an increase in protease levels resulting in the off-target destruction of critical proteins such as collagen, surface receptors/integrins, extracellular matrix proteins and growth factors resulting in prolonged healing and the potential for even greater bacterial growth. If bacterial presence and protease production are suspected, in the absence of deeper tissue infection, the use of an antimicrobial dressing following wound debridement can reduce wound colonization and ultimately prevent the formation/reformation of bacterial biofilm. Further, to address the presence of elevated protease levels, clinicians can utilize collagen dressings to sequester and bind up proteases as a sacrificial substrate protecting the proteins of the wound bed.
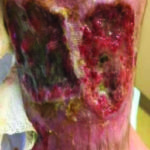 Figure 8. Ulcers 6 days after use of autolytic surfactant debridement
Figure 8. Ulcers 6 days after use of autolytic surfactant debridement
Implication of INFECTION/INFLAMMATION:Infection/inflammation is a critical point of management and likely the easiest missed as there may not be clearly visible evidence other than a non-healing wound, but another stimulus to reignite the inflammatory response.
M: MOISTURE
The optimization of a moist wound bed to promote wound healing has been in practice for close to four decades and is the driver for decision making related to dressing choices.19Insufficient moisture in exposed wound tissues causes desiccation and cell death, and prevents epithelial migration and matrix deposition; while excessive moisture due to exudate inhibits cell proliferation and breaks down matrix components.20 Moisture ties into WBP and the TIME concept as clinicians assess the source of the drainage and manage that source, be it bacterially driven as the stimulus for excessive drainage, or, for example, edema driven, making compression the important management option.
Implication of MOISTURE: Creating the optimal wound environment either by adding moisture or absorbing exudate is a critical balance in wound management, one that often changes throughout the course of treatment as the wound bed improves and moves toward a healing trajectory.
E: EPITHELIAL MIGRATION / EDGE OF WOUND
The last and final component to the TIME principle of wound healing is also the final marker of a healing wound, epithelial migration of cells across a well-prepared wound bed (see Figure 9). While this sounds obvious and easy, it is the activity that will not occur if the other principles have not been adequately addressed and one easily missed because the reasons may not be obvious on a visual exam. While the visible presence of slough, proteinaceous coagulum, exudates and residual dressing material may trigger a debridement or cleansing that may appear adequate, the continued presence of elevated proteases in an inflamed wound will be non-visible and should be assumed when epithelialization is not occurring. Additionally, the presence of microscopic biofilm will not allow epithelial cells to migrate and attach due to the absence of appropriate adhesion molecules, often resulting in thickened hyperkeratotic edges that then further need to be addressed.21 Lastly, the presence of callus or epiboly will require complete debridement in order to open up the edge, exposing the basement membrane to allow for migration of keratinocytes.
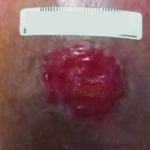 Figure 9. Epithelialization from the edge of the wound
Figure 9. Epithelialization from the edge of the wound
Implications of EDGE: Epithelial migration from the wound edge is the visible and measurable marker of wound closure. Attention to this important segment with each assessment allows for intervention possibly avoiding further and more invasive strategies later.
SUMMARY
Adequate wound bed preparation is a key factor in managing both acute and chronic wounds, second only to the proper diagnosis and subsequent appropriate supportive management of the wound such as offloading and compression. Clinicians utilizing the TIME concept can create an organized approach to addressing the common barriers to healing and restore balance to support the patient’s endogenous healing or facilitate the effectiveness of exogenous advanced therapeutic measures.
References
1.Krzyszczyk P, Schloss R, Palmer A, Berthiaume F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front Physiol. 2018;9:419.
2.Spear, M. Acute or chronic? What’s the difference? Plast Surg Nurs. 2013;33:98-100.
3.Falanga, V. Wound Bed Preparation and the Role of Enzymes: A Case for Multiple Actions of Therapeutic Agents. WOUNDS. 2002;14:47-57.
4.Enoch S., Harding K. Wound Bed preparation: The Science Behind the Removal of Barriers to Healing. WOUNDS. 2003;15:213-229.
5.Schultz GS, Sibbald RG, Falanga V, et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen. 2003; Supp1:S1-S8. https://doi.org/10.1046/j.1524-475X.11.s2.1.x
6.Leaper DJ, Schultz G, Carville K, et al. Extending the TIME Concept: What have we learned in the past 10 years? Int Wound J. 2012; 9(sup 2):1-19.
7.Cardinal M, Eisenbud DE, Armstrong DG, et al. Serial surgical debridement: A retrospective study on clinical outcomes in chronic lower extremity wounds. Wound Repair Regen. 2009;17:306-311. DOI: 10.1111/j.1524-475X.2009.00485.x
8.Moore Z. The important role of debridement in wound bed preparation. Wounds Int J. 2012;3:19-23.
9.Leaper D. Sharp technique for wound debridement. World Wide Wounds. http://www.worldwidewounds.com/2002/december/Leaper/Sharp-Debridement.html#. December 2002. Accessed June 1, 2018.
10.Strohal R, Apelqvist J, Dissemond J. et al. EWMA Document: Debridement. J Wound Care. 2013:22 (Suppl. 1): S1-S52.
11.Wound, Ostomy and Continence Nurses Society. (2015). Methods of Wound Debridement: Best Practice for Clinicians. Mt. Laurel: NJ
12.Santyl [package insert]. Andover, MA: Smith and Nephew Inc; 2013. http://www.santyl.com/content/pdf/SANTYL_Package_Insert-2013.pdf
13.Jovanovic A, Ermis R, Mewaldt R, et al. The influence of metal salts, surfactants, and wound care products on enzymatic activity of collagenase, the wound debriding enzyme. WOUNDS. 2012;24:242-253.
14.Weir D, Niezgoda J, Scarborough P. Wound Debridement. In: Krasner DL, Rodeheaver GT, Sibbald RG, eds. Chronic Wound Care: A Clinical Source Book for Healthcare Professionals. 4th edition. Malvern, Pa: HMP Communications; 2007:343-355.
15.Steed DL, Donohoe D, Webster MW, Lindsley L. Effect of extensive debridement and treatment on the healing of diabetic foot ulcers. Diabetic Ulcer Study Group. J Am Coll Surg. 1996;183:61-64.
16.Cowan LJ, Stechmiller JK, Phillips P, Yang QP, Schultz G. Chronic Wounds, Biofilms and Use of Medicinal Larvae. Ulcers. 2013;Article ID 487024, 7 pages; http://dx.doi.org/10.1155/2013/487024.
17.Wiegand C, Reddersen K, Hipler UC, et al. In vitro Evaluation of the Cleansing Effect of a Monofilament Fiber Debridement Pad Compared to Gauze Swabs. Skin Pharmacol Physiol 2016;29:318-323.
18.Percival SL, Mayer B, Malone M, et al. Surfactants and their role in wound cleansing and biofilm management. J Wound Care. 2017;26:680-690.
19.Winter GD. Formation of the scab and the rate of epithelialization of superficial wounds in the skin of young domestic pigs. Nature. 1962;193:293-294.
20.Ovington LG. Hanging wet to dry dressings out to dry. AdvSkin Wound Care. 2002;15:–:79-84.
21.Olczyk P, Mencner L, Komosinska-Vassev K. The role of extracellular matrix components in cutaneous wound healing. BioMed Res Int. 2014; 2014:747584. http://dx.doi.org/10.1155/2014/747584
22.Sibbald RG, Elliott JA, Avello EA, Somavali R. Optimizing the Moisture Management Tightrope with Wound Bed Preparation. Adv Skin Wound Care. 2015;28:466-476.

