Dr. Graham graduated with a baccalaureate degree in Biomedical Sciences from Grand Valley State University in 2009. He then went on to attain his Doctorate of Podiatric Medicine in 2014 from Barry University.Dr. Graham is currently in his 1st year of podiatric medicine and surgical residency with the added credential of reconstructive rearfoot/ankle surgical training (PMSR/RRA) at Jackson North Medical Center in Miami, Florida. He also serves as the president of the Podiatry Overseas Association, a non-profit medical mission organization.

Joey Ead is currently a first year medical student at Barry University School of Podiatric Medicine. Prior to attending medical school, Mr. Ead received his Masters Degree in biomedical science with a concentration in wound tissue repair. Mr. Ead has been involved in various research studies and has worked closely with wound care specialist Dr. Robert Snyder DPM, MSc, CWS.
Graham and Ead_Current Dialogues in Wound Management_2015_Volume 1_Issue 4
PATHOPHYSIOLOGY OF PERIPHERAL ARTERIAL DISEASE IN DIABETIC PATIENTS
Peripheral arterial disease (PAD) in patients with diabetes encompasses a global vascular problem. The two circulatory systems that affect arterial wounds are microvascular and macrovascular. Approximately 80% of patients with PAD have diabetes and a high risk of developing arterial wounds. Microcirculatory dysfunction, a functional rather than an obstructive process, along with thickening of the basement membrane, places patients with diabetes at a greater risk of nonhealing when ulcerations are present. The endothelial and smooth muscle cell vasodilatory response in patients with PAD is often damaged, inelastic, and not fully functional. Additionally, macrovascular PAD is a condition in which plaque thickens along the arterial tunica intima. Once enough plaque has calcified, arterial stenosis formation initiates, causing blood flow obstruction. Patients with diabetes frequently develop vascular disease below the knee (e.g., trifurcation or tibioperoneal disease), which has a deleterious effect on the flow of oxygen-rich blood reaching vital parts of the foot. Interrupted blood flow to the lower limbs can result in ischemia, hypoxia, pulselessness, paresthesias, and pain. If severe critical arterial stenosis or complete blockage occurs, the patient may be susceptible to tissue necrosis (gangrene), infection, sepsis, limb loss, and death (see Figure 1 and 2).
Some of the key risk factors for PAD include cigarette smoking, diabetes, hypertension, hyperlipidemia, obesity, coronary heart disease, old age, and metabolic syndrome. Heredity may also play a role. The wound is classically located distally, at the tips of toes or between the toes. The wound bed is covered with pale/necrotic tissue with minimal signs of exudate remnants. The wound edge usually appears “punched out,” and associated skin changes, such as shiny skin, can be observed. Increased endothelial and platelet activation, a prothrombotic state, affects patients with PAD. Multiple etiologies, including plaques, aneurysms, arteriosclerosis, hypercoagulable states or microembolic
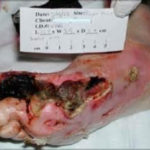 Figure1. First ray gangrene of the Right foot, secondary to macrovascular PAD
Figure1. First ray gangrene of the Right foot, secondary to macrovascular PAD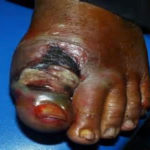 Figure 2. Wet gangrene, secondary to non-healing diabetic ulceration/infection, Left great toe. Patient with established microvascular PAD
Figure 2. Wet gangrene, secondary to non-healing diabetic ulceration/infection, Left great toe. Patient with established microvascular PAD
showering, and thrombotic episodes from atrial thrombosis may cause arterial lesions.
Arterial lesions are usually very painful, especially at night. Pulses are generally not palpable, although in patients with diabetes, the absence or presence of palpable pulses cannot be used as a determinant of a PAD diagnosis. No single test can determine arterial disease in an office setting, but Ankle Brachial Indices (ABI) and Toe Brachial Index (TBI) may be helpful. In diagnosing patients with arterial wounds attributed to PAD, the American Diabetes Association recommends ABI as an accurate marker for vascular evaluation. Diagnostic interpretation indicates that low ABI ratios are associated with a high vascular risk. Normal values range from .91 to 1.3, and ratios <.01 or >1.3 could indicate PAD. ABI may be falsely elevated in many of these patients, with readings >1.3, as vessels at the level of the ankle may be noncompressible secondary to medial calcinosis. Therefore, TBI may be more appropriate, since this phenomenon does not occur in the digits.
Ultimately, most of these patients require vascular consultation and often arteriography. This vascular diagnostic tool helps demonstrate the arterial tree in its entirety and readily delineates the site of arterial stenosis and occlusion.1 If there is a high clinical index of suspicion that a wound is ischemic, or for individuals with a high risk of PAD, a referral for secondary-tier evaluations may be appropriate. Investigations may include segmental pressure volume and skin perfusion pressure, transcutaneous oxygen measurement (TCPO2), and evaluation of lower limb indices and waveforms, such as segmental pressure volume recording.2 Two evidence-based reviews support the use of TCPO2 as a screening tool for a wound population with a high risk of vascular disease.2 A vascular consultation is imperative in these cases.
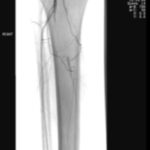
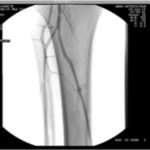
The importance of blood flow and oxygen delivery to a wound bed cannot be overstated. The angiosome concept represents a universal approach to evaluating vascularity in patients with diabetes. There are six angiosomes in the foot that originate from the three major arteries in the lower leg (posterior tibial, anterior tibial/dorsal pedis, and peroneal). Choke vessels mark the boundary of any angiosome and can supply blood to an adjacent angiosome through the delay phenomenon. The distal vessels at the level of the ankle (posterior tibial, anterior tibial, and peroneal) are often spared, making distal bypass and endovascular intervention possible. The angiosome idea conceptualizes the vascular redundancy of pedal blood flow and is an excellent way to determine which vessel interventions may create the most benefit. Many specialists still subscribe to bypass surgery as being more durable and, therefore, preferred for treating vascular disease in the lower extremities in patients with diabetes.2Endovascular intervention, such as angioplasty or stenting, may give patients a “window of opportunity” to heal previously chronic wounds in a minimally invasive manner; studies show this methodology is often sufficient to allow healing, particularly in patients who would not otherwise be candidates for extensive bypass surgery (Figure 3).
Figure 3. Arteriograms taken before and after endovascular intervention. Arteriography is considered to be the gold standard for the evaluation of atherosclerosis, and improvement of blood flow is paramount in treating patients with arterial obstruction. Images courtesy of Dr. Peter Swischuk, Chief of Interventional Radiology, Jackson North Medical Center in North Miami Beach, Florida.
TREATMENT OF ARTERIAL WOUNDS IN THE DIABETIC PATIENT
Once vascularity has been improved, wound care in this patient group requires a multidisciplinary approach and comprehensive wound bed preparation (WBP) including debridement, if feasible; control of infection and inflammation; moisture balance; and wound edge preparation.4 The WBP concept is holistic and includes determining wound etiology and addressing patient-centered concerns.
SUMMARY
Assessment of diabetic arterial wounds should encompass a comprehensive lower extremity evaluation and a thorough understanding of the patient’s medical history. Clinicians should incorporate a multidisciplinary approach in the treatment of this condition. It is widely understood that healing of ischemic wounds is more likely when proper arterial perfusion is reestablished across the foot and ankle. Management of these wounds should include pain control, infection management, conservative surgical debridement, proper dressings, and improvement of circulation. Lifestyle changes should be recommended to patients who smoke, live a sedentary lifestyle, and are morbidly obese. Clinicians with a strong understanding of the biochemical processes and advanced vascular knowledge are more likely to have success in healing these wounds.
References
1.Spentzouris G, Labropoulos N. The evaluation of lower-extremity ulcers. Semin Intervent Radiol. 2009;26(4):286-95.
2.Snyder RJ, Kirsner RS, Warriner RA III, et al. Consensus recommendations on advancing the standard of care for treating neuropathic foot ulcers in patients with diabetes. Ostomy Wound Manage. 2010;56:S1-24.
3.Tefera G, Hoch J, Turnipseed WD. Limb-salvage angioplasty in vascular surgery practice J Vasc Surg. 2005;41:988-93.
4.Donegan RJ, Schmidt BM, Blume PA. An overview of factors maximizing successful split-thickness skin grafting in diabetic wounds. Diabet Foot Ankle. 2014; 5:10.3402/dfa.v5.24769