
Dr. Robert Snyder is a podiatrist with over 30 years of experience; his practice is limited to wound management and limb preservation. He is Professor and Director of Clinical Research and Fellowship Director at Barry University SPM. Dr. Snyder is certified in foot and ankle surgery by the American Board of Podiatric Surgery and is also a board certified wound specialist. Dr. Snyder is immediate past-president of the Association for the Advancement of Wound Care and past-president of the American Board of Wound Management. In addition to his doctorate, he holds an MSc in Wound Healing and Tissue Repair from Cardiff University. Dr. Snyder has published several book chapters over 125 papers in peer reviewed and trade journals on wound care and has been a principal investigator on more than 30 randomized controlled trials.
Snyder_Current Dialogues in Wound Management_2016_Volume 2_Issue 3
NOTE: As with any case study, the results and outcomes should not be interpreted as a guarantee or warranty of similar results. Individual results may vary depending on the patient’s circumstances and condition.
Chronic wounds represent a significant medical condition that exponentially increases disability, mortality, and costs. Complex ulcers with exposure of vital structures such as bone, tendon, and muscle represent major challenges for wound care physicians. These sequelae frequently lead to amputations in patients with diabetes. Standard wound management (i.e., moist wound healing, debridement, and offloading) often fails to prevent desiccation of these critical structures. Further need for multiple operating room interventions often results in compromised function and ultimate limb loss.
Human skin allografts have traditionally been utilized in the treatment of patients with burns. However, a 12-week, prospective study by Reyzelman et al showed that treatment with an acellular dermal regenerative matrix healed diabetic foot ulcers 2.7 times faster than those in the standard of care group.1 This modality has also been used for complex wounds by aiding in the formation of granulation tissue while keeping vital structures moist and viable.2(Figures 1-4)
Research consistently reports many potential benefits of allograft skin.3 This therapy:4
1.limits infection and keeps the wound bed mechanically clean;
2.decreases water, electrolyte, and protein loss5;
3.decreases energy requirements;
4.reduces pain, allowing exercise and ambulation while decreasing the incidence of contractures;
5.conserves autograft;
6.improves appetite and general welfare;
7.improves patient’s psychological outlook;
8.decreases necessity for flap repair
Snyder and Simonson6 researched cadaveric allografts as adjunctive therapy for non-healing ulcers of multiple etiologies. The benefits of allografts remained particularly noteworthy when applied to wounds exhibiting exposed bone and/or tendon. Granulation tissue often completely covered these structures after allograft slough or incorporation. Granulation tissue was apparent through the graft fenestrations at an average of 13.4 days. It was further observed that cadaveric allografts prevented desiccation, controlled infection, promoted granulation tissue, and reduced pain in non-healing wounds.
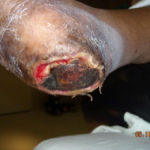 Figure 1. Complex pressure ulcer in a patient with diabetes
Figure 1. Complex pressure ulcer in a patient with diabetes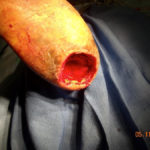 Figure 2. CS/P excision of the ulceration revealing the underlying calcaneus
Figure 2. CS/P excision of the ulceration revealing the underlying calcaneus
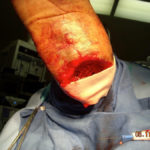 Figure 3. Beginning the process of cadaveric allograft application
Figure 3. Beginning the process of cadaveric allograft application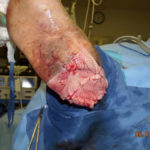 Figure 4. Completed application of fenestrated cadaveric allograft. NPWT was applied over the graft and a non-adherent interface
Figure 4. Completed application of fenestrated cadaveric allograft. NPWT was applied over the graft and a non-adherent interface
A prospective study involving 15 patients with complex wounds treated with biologically active cryopreserved human skin allograft was performed by Wilson et al.2 The mean duration of days until coverage of bone and/or tendon with granulation tissue was 5.16 weeks (range 5-117 days). Ninety-three percent of all wounds healed completely in 19 weeks (range 53-311 days). Researchers found this modality both safe and effective in treating complex wounds with exposure of vital structures.
Oliver et al7 observed that allograft skin adhered to the wound surfaces rather than acting as a mechanical covering. This dynamic provided a physiologic environment against microbiological invasion while allowing host defenses to control bacterial proliferation.8Histologically, cadaveric allograft does not “take” in the traditional sense. Researchers postulate that granulation tissue actually replaces the graft while providing an appropriate matrix for epithelial relining.
Cadaveric allograft has successfully been used in combination with other advanced therapeutic modalities: the results may be synergetic. These include multilayered compression,9 topical growth factors10 and hyperbaric oxygen.11 Findings from a study conducted by Kashefski and Marton suggest that combining advanced healing modalities with Total Contact Casting (TCC) may also create synergism in reducing times to heal.12The macro- and microstrain mechanisms of Negative Pressure Wound Therapy (NPWT) appear to produce a particularly significant effect when combined with cadaveric allograft. Removing wound fluid decreases bacterial burden and aids in eliminating hematoma and seroma under the graft. Furthermore, this device stabilizes and bolsters the graft. Increased nutrition through improved circulation may function synergistically through augmentation of healing.13Anecdotally, NPWT with instillation may also be of benefit.14 In wounds that don’t completely re-epithelize; therapies such as split thickness skin grafts (STSG) or epidermal blister grafts may be of benefit once vital structures are covered.
CONCLUSION
Complex wounds often represent a conundrum for wound healing professionals. The utilization of cadaveric allograft may be beneficial in granulating over bone, tendon, or muscle. Employment with modalities such as NPWT may have a synergic effect in promoting healing and/or encouraging wound bed preparation.
References
1.Reyzelman A, Crews RT, Moore JC, et al. Clinical effectiveness of an acellular dermal regenerative tissue matrix compared to standard wound management in healing diabetic foot ulcers: a prospective, randomized, multicenter study. International Wound Journal 2009;6(3): 196-208.
2.Wilson TC, Wilson JA, Crim B, Lowery NJ. The use of cryopreserved human skin allograft for the treatment of wounds with exposed muscle, tendon, and bone. WOUNDS 2016;28(4): 119-125.
3.Makie D. Postal survey on the use of glycerol-preserved allografts in clinical practice. Burns. 2002;28(Suppl 1): S40-4.
4.Snyder RJ. Treatment of nonhealing wounds with allografts. Clinics in Dermatology 2005;23: 388-395
5.Salisbury RE, Carnes RW, Enterline D. Biological dressings and evaporative water loss from burn wounds. Annals of Plastic Surgery1980; 5: 270-2.
6.Snyder RJ, Simonson DA. Cadaveric allograft as adjunct therapy for non-healing ulcers. Journal of Foot Surgery 1999;38: 93-101.
7.er AM, Kaawach W, Mithoff EW, et al. The differentiation and proliferation of newly formed epidermis on wounds treated with cultured epithelial allografts. British Journal of Dermatology 1991;125: 147-154.
8.Wong L. The many uses of allograft skin. Ostomy Wound Management. 1995:41: 36-42.
9.Reno F. In vitro mechanical compression induces apoptosis and regulates cytokines release in hypertrophic scars. Wound Repair and Regeneration 2003;11: 331-6.
10.Snyder RJ. The use of cadaveric allograft and recombinant human platelet-derived growth factor-bb (becaplermin): a potentially synergic effect for accelerated healing of recalcitrant wounds. Poster presentation, Wound Healing Society 2000 Educational Symposium, Toronto, Canada, June 4-6, 2000.
11.Bartlett R. Hyperbaric oxygen therapy. Presented at the New Cardiovascular Horizons Symposium, New Orleans, LA, October 23-25, 2003.
12.Kashefski H, Marton W. Total contact casting combined with human fibroblast-derived tissue in 15 DFU patients. Journal of Wound Care 2012;2:21.
13.Armstrong DG, Lavery LA, Abu-Rumman P. Outcomes of sub atmospheric pressure dressing therapy on the wounds of the diabetic foot. Ostomy Wound Management 2002;48: 64-8.
14.Lessing C, Slack P, Hong KZ, et al. Negative pressure wound therapy with controlled saline instillation (NPWTi): dressing properties and granulation response in vivo. Wounds 2011;23(10):309-319.

