Before joining Acelity, Ajay Chaudhary enjoyed a varied career in the corporate world, in a nongovernmental organization, and in healthcare. In the corporate world, he has acted as a regional medical advisor for a pharmaceutical company and a clinical resource consultant for a medical device company. He has also managed teams of healthcare professionals involved in community outreach for an international corporation and in healthcare information technology development. In addition, Dr. Chaudhary has spent time working with a global health agency and as a medical officer in a variety of hospital settings, including emergency and intensive care.
Chaudhary_Current Dialogues in Wound Management_2015_Volume 1_Issue 3
INTRODUCTION
The CelluTome™ System was introduced to the Indian subcontinent in November 2013. The concept behind the product was unique in India and received a range of reactions—from interest to skeptical curiosity. Transitioning from split-thickness skin grafting to epidermal grafting for wounds was a therapeutic concept that required a market development effort.
The CelluTome™ System is an automated epidermal harvesting system from KCI (an Acelity company, San Antonio, TX). The device, which is applied with a harvester over the donor site (usually the medial aspect of the thigh), uses controlled negative pressure (-400 mm Hg to -500 mm Hg) and warmth (37°C to 41°C) to raise epidermal microdomes that are then harvested once dome formation is complete. The entire process typically takes 30 to 45 minutes; the grafts obtained are uniform and can be transferred directly to the graft site using a 3M™ Tegaderm™ film (3M Company) or ADAPTIC TOUCH® Non-Adhering Silicone Dressing (Systagenix Wound Management, Limited, an Acelity company, Gargrave, UK). Wound re-epithelialization is monitored at each dressing change and at weekly follow-up visits, and wounds are considered healed when complete closure is observed.
SUCCESSES
Initially, surgeons were concerned about the performance of the system for patients of different ethnicities, with different comorbidities, and other factors. We shared the results of our prelaunch studies in India, which were conducted over 8 to 12 weeks. Grafts obtained with the CelluTome™ System were applied on subjects with different indications, from chronic wounds to burns. Initial variations in graft response were minimized with a standardized harvesting, application, and dressing protocol. Epidermal grafting was found to be ideal for wounds that were 10 × 10 cm2 or smaller, with mild-to-moderate exudate. The presence of healthy granulation tissue was crucial to cell proliferation and wound closure, and we found this could be optimized by managing the wound with negative pressure wound therapy and one or two V.A.C® Therapy dressings before epidermal grafting.
The harvesting procedure requires a CelluTome™ Therapy Unit, 1 single-use Harvester for each set of epidermal grafts harvested, a suitable dressing for graft transfer, such as the ADAPTIC TOUCH® Non-Adhering Silicone Dressing, and after 1 week, a nonadhesive follow-up dressing. Bolster dressings are recommended to optimize cell proliferation and prevent shearing of the grafts.
In a case series (n=18) conducted in South India, 88.9% of wounds healed completely after epidermal grafting, with complete wound closure seen by 5 to 6 weeks in 16 patients, and a mean duration to epithelialization of 3.7 weeks. All donor sites healed without complications (unpublished data).
DISCUSSION
Since its introduction in 2013, epidermal grafting using grafts obtained with the CelluTome™ System has become increasingly accepted by vascular and plastic surgeons. The simple process for graft harvesting requires no anesthesia, produces minimal or no pain at the donor site, and provides uniform size and thickness of viable grafts. In addition, continuous patient monitoring is not required. These factors all contributed to initial use and acceptance of the CelluTome™ System by surgeons. Development of a guideline specifically for the Indian subcontinent supported better wound selection and wound bed preparation for improved cell proliferation. Inclusion of critical points for follow-up dressing changes at the graft site also enhanced the success of epidermal grafting. Any patient with a wound needing epidermis was a suitable candidate; knowledge of the risk factors for graft rejection helped mitigate these risks by ensuring appropriate management, such as better glycemic control, improved nutritional status, and use of V.A.C.® Therapy before grafting. In some older patients, dome formation may occur within 15 minutes, and the grafts can be harvested and applied immediately after microdome formation.
Our customers have also expanded their use of the CelluTome™ System to harvesting grafts for esthetic applications, such as patients with stable vitiligo and other pigmentation disorders
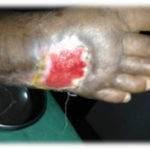
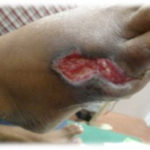
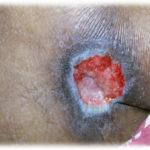
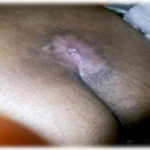
Figure 1. This patient with diabetes mellitus was treated with insulin, metformin 1 gm od, and glibenclamide 5 mg bid. The patient developed a diabetic foot ulcer on the dorsum of the right foot; treatment included empiric broad-spectrum antibiotic therapy. The photographs illustrate the appearance of the wound at the time of grafting (A) and after 40 days (B).
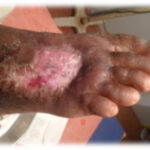
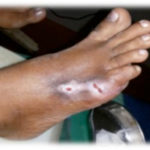
Figure 2. This ulcer in a patient with rheumatoid arthritis required no antibiotic therapy. The photographs illustrate the appearance of the wound at the time of grafting (A) and after 40 days (B).
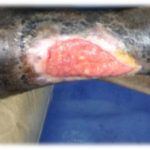
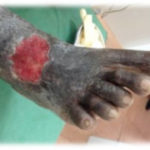
Figure 3. This patient with diabetes mellitus was treated with insulin and metformin 1 gm od. The patient developed a diabetic foot ulcer on the lateral dorsal right foot and received empiric broad-spectrum antibiotic therapy. The photographs illustrate the appearance of the wound at the time of grafting (A) and after 40 days (B).
REPRESENTATIVE CASE STUDIES
The following photographs, taken from the unpublished case series conducted in South India illustrate epidermal grafting outcomes using grafts harvested with the CelluTome™System in several patients with ulcers of varying etiology.
NOTE: As with any case study, results and outcomes should not be interpreted as a guarantee or warranty of similar results. Individual results may vary depending on the patient’s circumstances and condition.
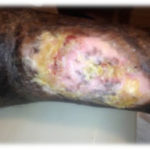
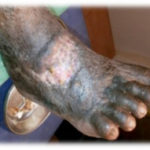
Figure 4. This patient with diabetes mellitus was treated with insulin, metformin 1 gm od, and glibenclamide 5 mg bid. The patient also received antihypertensive therapy with losartan 50 mg od and amlodipine 5 mg od. The patient developed a diabetic foot ulcer on the dorsum of the right foot and received empiric broad-spectrum antibiotic therapy. The photographs illustrate the appearance of the wound at the time of grafting (A) and after 40 days (B).
Figure 5. This patient had traumatic paraplegia and diabetes mellitus treated with insulin and metformin 1 gm od. The patient developed a sacral pressure ulcer and received empiric broadspectrum antibiotic therapy. The photographs illustrate the appearance of the wound at the time of grafting (A) and after 40 days (B).