Dr. Robert Snyder is a podiatrist with over 30 years of experience; his practice is limited to wound management and limb preservation. He is Professor and Director of Clinical Research and Fellowship Director at Barry University SPM. Dr. Snyder is certified in foot and ankle surgery by the American Board of Podiatric Surgery and is also a board certified wound specialist. Dr. Snyder is immediate past-president of the Association for the Advancement of Wound Care and past-president of the American Board of Wound Management. In addition to his doctorate, he holds an MSc in Wound Healing and Tissue Repair from Cardiff University. Dr. Snyder has published several book chapters over 125 papers in peer reviewed and trade journals on wound care and has been a principal investigator on more than 30 randomized controlled trials.


Joey Ead is currently a second year medical student at Barry University School of Podiatric Medicine. Prior to attending medical school, Mr. Ead received his Masters Degree in biomedical science with a concentration in wound healing and tissue regeneration. Mr. Ead has been involved in various research studies and has worked closely with wound care specialist Dr. Robert Snyder DPM, MSc, CWS.
Snyder, Cuffy and Ead_Current Dialogues in Wound Management_2017_Volume 3_Issue 1
NOTE: As with any case study, the results and outcomes should not be interpreted as a guarantee or warranty of similar results. Individual results may vary depending on the patient’s circumstances and condition.
OVERVIEW
Charcot neuropathic osteo-arthopathy (CN), commonly known as Charcot foot, is an uncommon syndrome that typically manifests in the diabetic patient. In spite of distinct pathognomonic features, this deformity is commonly misdiagnosed. Common pathologies mimic CN consequently deviating the clinician from making the correct diagnosis. The Charcot foot is a significant lower extremity complication that can ultimately result in bone/joint deformity, ulceration and increase the possibility of limb loss.
EPIDEMIOLOGY
CN currently affects only 1% of diabetics1,2. However it has been reported in 29% of diabetic patients who present with peripheral neuropathy and a loss in proprioception1,2. The survival rates for diabetic amputations at 5 years are only 50%
PREDISPOSITIONS
Charcot syndrome may manifest itself in patients with a wide spectrum of unrelated diseases stemming particularly from nerve damage. This pathology may be exacerbated or activated by:
• Toxins and or infections4
• Spinal cord diseases (tabes dorsalis, syringomyelia)4
• Parkinson’s4
• HIV4
• Sarcoidosis4
• Rheumatoid disease
Peripheral polyneuropathy is a common manifestation caused by chronic alcoholism10. Nutritional deficiencies in thiamine secondary to alcohol toxicity can damage the integrity of the peripheral nervous system (autonomic, motor and sensory elements)10. This can cause paraesthesias, pain, polyneuropathy and weakness in the lower extremity making these patients susceptible to CN
Loss of proprioceptive sensation disrupts the body’s biomechanical gait cycle that leads to abnormal loading4. This disruption can cause joint collapse and fractures within compromised areas of the lower extremity4. Motor neuropathy could potentially result in foot and ankle structural deformation (overstressed plantar arch – distal clawing)
PATHOPHYSIOLOGY
The pathogenesis of CN is based on a series of complex biochemical, biomechanical and physiologic processes. Autonomic neuropathy disrupts the integrity of the smooth muscle tonus on the arterial wall3. This leads to inadequate vaso-regulation and an increase in blood flow to the bone3. Consequently, osteoclasts and monocytes storm the affected site causing an increased bone resorption rate that eventually results in osteopenia3. This makes lower limb structures more susceptible to dislocations, fractures, and joint collapses. It has been found that the insensate foot is more prone to repetitive unrecognized traumas due to abnormal gait cycles3. Chronic inflammation to the damaged site triggers a cascade of events that ultimately leads to CN4. The homeostatic balance between anti and pro inflammatory cytokines that help maintain the inflammatory process is compromised4. The figure below will give clinicians an idea on the mechanism behind the “RANKL-RANK” pathway. (Figure 1)
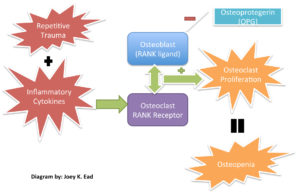 Figure 1. RANKL – RANKPathways: The binding of RANK Ligand to the RANK receptor is an essentioal component of osteoclast proliferation and bone resorption. The diminished function of the osteoblast derivative OPG hinder its intrinsic ability to regulate bone formation and resorption.
Figure 1. RANKL – RANKPathways: The binding of RANK Ligand to the RANK receptor is an essentioal component of osteoclast proliferation and bone resorption. The diminished function of the osteoblast derivative OPG hinder its intrinsic ability to regulate bone formation and resorption.
DIAGNOSIS
Diagnostic modalities include: X-rays, CT, bone scans, and MRI. Imaging is an integral factor in the early and diagnostic phases of CN11. It should be noted that MRI’s are the most sensitive image study in the detection of early/acute stages of CN11. Additionally, MRI’s may be able to help clinicians distinguish and differentiate between acute neuro-arthropathy from acute osteomyelitis11. Other testing may include Ceretec or Indium white blood cell scans. While a triple phase bone scan lacks specificity in the diagnosis of osteomyelitis, it may be used in conjunction with other image studies (MRI, Ceretec) for dual peak imaging16. However, despite the specificity and sensitivity of several of these techniques, a diagnosis of Charcot versus osteomyelitis remains challenging especially in the presence of ulceration.
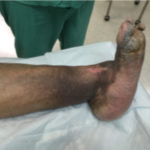 Figure 2. CN patient
Figure 2. CN patient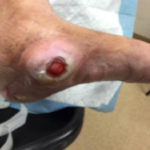 Figure 3. CN patient who presents an ulceration on the medial aspect of the midfoot.
Figure 3. CN patient who presents an ulceration on the medial aspect of the midfoot.
Initial symptoms and manifestations of Charcot syndrome maybe mild but can become significant if unperceived repetitive trauma remains unabated. Key diagnostic – pathognomonic features should include the following components for abnormalities:
•Vascular4
•Neurological4
•Radiographic4
•Musculoskeletal
Some early clinical findings include a warm, swollen and erythematous foot with discomfort and pain in an otherwise insensate foot4. Most of the affected population with Charcot syndrome will present with well-preserved vascularity or even amplified arterial blood flow at the designated region4. The “washed out” appearance of the bones may be directly correlated to this phenomenon. Due to overlapping symptomatic features, early clinical symptoms can be misdiagnosed as deep vein thrombosis, infection, or acute gout among others4. Charcot syndrome patients typically display the classic “rocker-bottom” foot with or without plantar ulceration (Figure 2,3) 4. Charcot foot is most commonly seen in the mid-foot, however can occur in the digits, forefoot, and ankle. The Eichenholtz classification system is one of the most utilized protocols to pinpoint the evolution of this syndrome based on key pathologic observations (Figure 4).
CONSERVATIVE TREATMENT
Conservative management often remains an effective option. Treatment of the patient should factor in the stage of Charcot syndrome, site(s) of involvement, presence or absence of chronic ulceration, presence or absence of infection, overall medical status, and level of compliance
OFFLOADING
In cases of acute Charcot foot, offloading is paramount17. The Charcot Foot in Diabetes Consensus Report advocates immobilization and offloading at the acute stage of CN to prevent progression of deformity12. It is recommended that non-weight bearing cast
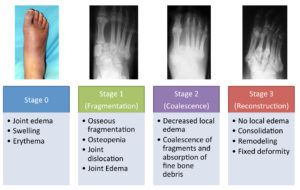 Figure 4. The Eichenholtz Classification System
Figure 4. The Eichenholtz Classification System
Pictures from: http://www.orthobullets.com/foot-and-ankle/7047/diabetic-foot-charcot-neuropathy
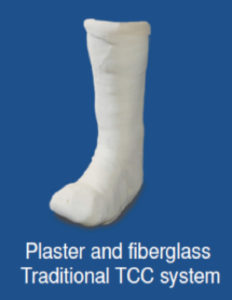
immobilization be administered for at least 3 months5. If available, an irremovable total contact cast (TCC) should be applied and then replaced after three days4 (Figure5). Any edema present may be substantially reduced due to the compressive forces of the dressing4 and these dressings may subsequently be changed weekly or as needed. Other offloading modalities could include the instant TCC (iTCC) that converts a removable cast walker (RCW) to one that is less easily removed (Figure 6)4,12. In a randomized control trial, 50 patients with diabetic foot ulcerations were randomly assigned in one of two offloading treatments groups: (RCW) or the same RCW wrapped with cohesive bandage (iTCC) 7. The subjects were evaluated for 12 weeks or until complete epithelialization was evident 7. By week 12, the
Figure 5: Total Contact Cast
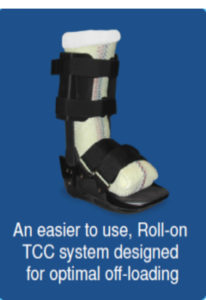
iTCC group had a healing rate of 82.6% versus the RCW group, which had a 51.9% healing rate 7. Additionally, the iTCC group healed significantly quicker than the latter (41.6 ±18.7 vs. 58.0 ± 15.2 days, P = 0.02) 7. The Charcot Restraint Orthotic Walker (CROW) may also be utilized during the acute phase of this syndrome (Figure 7) 12. Some key advantages of the CROW include: the ability to manage edema, minimize joint stiffness and created accessibility to inspect and treat ulcerations that may be affecting CN patients15. However, because this is a removable device, patients should be extensively counseled as to the importance of strict adherence.
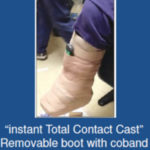 Figure 6. Instant Total Contact Cast
Figure 6. Instant Total Contact Cast
PHARMACOLOGIC THERAPIES
Due to the excess bone turn over in patients with active Charcot syndrome, anti-resorptive drugs are frequently included in the treatment protocol. However, there is currently no conclusive evidence supporting the use of agents such as oral bisphosphonates in the active Charcot foot. However, research has found the utilization of intravenous bisphosphonate therapy (i.e. Pamidronate) lead to a reduction in bone turnover in the diabetic CN patient13. Intranasal calcitonin (IC) therapy is another viable option8. Some advocates have opined the pharmacologic properties of IC are more efficacious than bisphosphonates because it has direct action on the RANKL/osteoprotegerin pathway6,8(Figure 1). More clinical trials are required to further elucidate the mechanism of action behind anti-resorptive therapy and future benefits.
ADVANCED MEDICAL DEVICES
Wound care guidelines, quality measures and pay for performance are now at the forefront of the health care industry and will translate to reimbursement pathways12. Unfortunately, only few of these guidelines address patient centered concerns (i.e. nutrition, vascularity and comorbidities)14. Patient history and etiology are critical factors when tackling chronic wounds. The DIME model incorporates key principles for the assessment and treatment of chronic wounds by tackling the etiology and biochemistry of wound healing14. This model encompasses robust wound bed preparation methods that accelerate endogenous healing and help eliminate factors that impede the wound-healing cascade14. This strategy includes; removal of devitalized tissue, control of infection/inflammation, moisture balance (exudate), and the need for wound edge preparation and depth management14. The DIME model utilizes active and passive treatments that approach wound bed preparation by including advanced therapies such as ORC/Collagen, negative pressure wound therapy (NPWT), NPWT with instillation, epidermal blister grafts, and hyperbaric oxygen therapy among others14. According to the Charcot consensus panel, advanced wound care therapies that require daily or frequent applications may not be suitable for use with TCC or other non-removable devices
 Figure 7. Charcot Restraint Orthotic Walker
Figure 7. Charcot Restraint Orthotic Walker
Electrical bone stimulation has been used to foster osteogenesis in the early stages of the pathology. Cell culture studies have shown that low energy electromagnetic fields stimulate insulin-like growth factor II6. This may create an intrinsic ability to increase calcium flux and bone cell proliferation6. It should be emphasized that the magnitude of the actual benefit is not clearly understood6,9. Even though these studies are promising, its use has been supported only as an adjunct therapy mainly after the postsurgical phase of treatment
CONCLUSION
Conservative treatment remains challenging for CN but has evolved over the years. Immobilization and mechanical protection is essential in order to prevent further collapse and permanent deformation in the Charcot foot. There are a variety of conservative treatment options; however additional evidence-based studies are warranted. All treatment modalities should be tailored to the patient based on the current state of their Charcot foot, medical status, and compliance tendencies. In the event that conservative treatment is not sufficient, surgical reconstruction may be necessary to restore functionality.
References
1.Zgonis T, Stapleton J, Roukis T. Charcot Foot and Ankle Deformity. McGlamry’s Comprehensive Textbook of Foot and Ankle Surgery. 4th ed, Ch 70. 1008-1021.
2.Cala M, Willis B, Carbonell B, Boyton S. Charcot Foot Limb Salvage Procedure with External Fixation and Medial Column Lengthening: A Case Presentation. The Foot and Ankle Online Journal 6 (8): 1
3.Kaynak, G., Birsel, O., Güven, M., & Öğüt, T. (2013). An overview of the Charcot foot pathophysiology. Diabetic Foot & Ankle,
4.doi:http://dx.doi.org/10.3402/dfa.v4i0.211174.Rogers LC, Frykberg RG, Armstrong DG, et al. The Charcot foot in diabetes. Diabetes Care. 2011;34(9):2123-2129.
5.Ramanujam CL, Facaros Z. An overview of conservative treatment options for diabetic Charcot foot neuroarthropathy. Diabetic Foot & Ankle. 2011;2:10.3402/dfa.v2i0.6418. doi:10.3402/dfa.v2i0.6418.
6.Ramanujam CL, Facaros Z. An overview of conservative treatment options for diabetic Charcot foot neuroarthropathy. Diabetic Foot & Ankle. 2011;2:10.3402/dfa.v2i0.6418. doi:10.3402/dfa.v2i0.6418.
7.Armstrong, D.G., Lavery, L.A., Wu, S., Boulton, A.J. Evaluation of removable and irremovable cast walkers in the healing of diabetic foot wounds: a randomized controlled trial. Diabetes Care. 2005;28:551–554.
8.Bem R, Jirkovska A, Fejfarova V, et al. Intranasal calcitonin in the treatment of acute Charcot neuroosteoarthropathy. Diabetes Care. 2006;29(6):1392-4.
9.Hanft, J.R., Goggin, J.P., Landsman, A., Surprenant, M. The role of combined magnetic field bone growth stimulation as an adjunct in the treatment of neuroarthropathy/Charcot joint. an expanded pilot study. J. Foot Ankle Surg. 37:510–515, 1998.
10.Arapostathi, C., Tentolouris, N., & Jude, E. B. (2013). Charcot foot associated with chronic alcohol abuse. BMJ Case Reports, 2013, bcr2012008263. http://doi.org/10.1136/bcr-2012-008263
11.Ergen, F. B., Sanverdi, S. E., & Oznur, A. (2013). Charcot foot in diabetes and an update on imaging. Diabetic Foot & Ankle, 4,10.3402/dfa.v4i0.21884. http://doi.org/10.3402/dfa.v4i0.21884
12.Snyder RJ, Frykberg RG, Rogers LC, Applewhite AJ, Bell D, Bohn G, et al. The Management of Diabetic Foot Ulcers Through Optimal Off-Loading. J Am Podiatr Med Assoc. 2014;104(6):555–567. doi: 10.7547/8750-7315-104.6.555.
13.Selby PL, Young MJ, Boulton AJM: Bisphosphonates: A new treatment for diabetic Charcot neuroarthropathy? Diabet Med 11:28-31, 1994.
14.Snyder, R. J., Fife, C., & Moore, Z. (2016). Components and Quality Measures of DIME (Devitalized Tissue, Infection/Inflammation, Moisture Balance, and Edge Preparation) in Wound Care. Advances in Skin & Wound Care, 29(5), 205–2
15. http://doi.org/10.1097/01.ASW.0000482354.01988.b415.Snyder, R. (n.d.). Diabetes: Offloading difficult wounds. Retrieved November 29, 2016, from http://lermagazine.com/article/diabetes-offloading-difficult-wounds
16. Snyder RJ, Kirsner RS, Warriner RA 3rd, Lavery LA, Hanft JR, Sheehan P. Consensus recommendations on advancing the standard of care for treating neuropathic foot ulcers in patients with diabetes. Ostomy Wound Manage 2010;56(4 Suppl):S1–24.