Professor & Director of Clinical Research, Barry University SPMDr. Robert Snyder is Professor and Director of Clinical Research and Fellowship Director in wound care and research at Barry University SPM. He has created and implemented the first post residency Wound Management and Research Fellowship for podiatric medical schools. He is certified in foot and ankle surgery by the American Board of Podiatric Surgery and is also a board certified wound specialist. Dr Snyder is past-president of the Association for the Advancement of Wound Care and past-president of the American Board of Wound Management, the certifying body for Wound Care Specialists. In addition to his doctorate, he holds an MSc in Wound Healing and Tissue Science from Cardiff University. His expertise at Cardiff, Wales, was further acknowledged as he was selected as an Internal Marker for MSc dissertations with an Honorary Title. To constantly expand his knowledge and stay current in all aspects of healthcare, he is completing an MBA in Health Management. Dr. Snyder is a key opinion leader and sought after speaker, lecturing extensively throughout the United States and abroad. He was chosen to develop and teach a wound care course for physicians internationally. Dr. Snyder has published several book chapters and over 150 papers in peer reviewed and trade journals on wound care. He serves on the editorial advisory boards of Ostomy Wound Management, Wounds and Podiatry Management and has recently been appointed as a periodic reviewer for the Lancet and NEJM. He has been a Principal Investigator on more than 50 randomized controlled trials for innovative wound healing modalities and products.

Dr. Cherison Cuffy is a graduate of Temple University School of Podiatric Medicine. He then completed a three-year podiatric medicine and surgery residency at Northwest Medical Center in Margate Florida. Dr Cuffy is currently faculty at Barry University School of Podiatry as an assistant professor. He now participates in the clinical education of the podiatry students along with a focus on clinical research at the Paul and Margaret Brand Research Center.

Joey Ead is currently a second year medical student at Barry University School of Podiatric Medicine. Prior to attending medical school, Mr. Ead received his Masters Degree in biomedical science with a concentration in wound healing and tissue regeneration. Mr. Ead has been involved in various research studies and has worked closely with wound care specialist Dr. Robert Snyder DPM, MSc, CWS.

Karen is a third year medical student at Barry University School of Podiatric Medicine.
Snyder, Cuffy, Ead and Fischborn_Advanced Wound Dressings Today_2017_Spring Supplement
A wound is a disruption of normal anatomic structure and function that is usually inclusive of the skin. Wound healing represents a comprehensive series of physiological and biochemical reactions that create an orderly healing cascade. This process includes four key phases: hemostasis, inflammation, proliferation and remodeling. Wound healing is a dynamic response that progresses along a continuum that should result in the restoration of anatomical and function integrity.
HEMOSTASIS
Any injury extending beyond the boundaries of the epidermis will cause bleeding. This phenomenon activates a series of overlapping events designed to control blood loss, seal the defect, and establish bacterial control. The disruption of blood vessels and exposure of the sub-endothelial environment (collagen) catalyzes platelet aggregation.1 Furthermore injured cells trigger both extrinsic and intrinsic coagulation pathways. Clot formation protects and seals the disrupted vessels so that blood loss is controlled.
Clot formation serves several key functions including:1
Temporary bacterial barrier
Interim matrix/scaffold for migrating cells
Reservoir of growth factors
Fibrinolysis is a vital process that occurs after clot formation. Platelet degranulation releases an assortment of energy producing cytokines and growth factors.1 The inflammatory phase is initiated by several growth factors including PDGF, IGF-I, EGF, TGF-β and TNF alpha.1
INFLAMMATION
Once the hemostatic process is finalized, secretion of pro-inflammatory cytokines and proteases (elastase/collagenase) help remove damaged extracellular matrix (ECM).2 The ECM gives skin its unique properties of elasticity, tensile strength, and compressibility. In acute wounds the provisional wound matrix (Fibrin; fibronectin) provides a scaffolding to direct cells into the injury and stimulate proliferation, differentiation, and synthesis to form a new ECM.
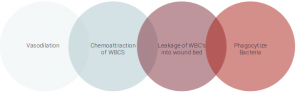
Before a wound can rebuild its damaged apparatus, breakdown of devitalized tissue and elimination of excess bacteria permeating the wound bed is paramount.2 Leukocyte migration out of the vessels and into the wound bed is conducted via diapedsis.1Teams of white blood cells (WBC) infiltrate the wound environment to help stabilize the bacterial environment and establish a clean wound bed (Figure 1).2 The principal leukocyte involved during this process is polymorphonuclear cells (PMN) also known as neutrophils. Their primary role is to eliminate deleterious bacteria and foreign debris via phagocytosis. Cell adhesion molecules (CAM) promote the binding mechanism between neutrophils and damaged tissues. Neutrophils release essential growth factors that attract additional leukocyte support. By days 3 to 4 after tissue damage, neutrophils disappear via apoptosis and are replaced by activated macrophages.1 They continue to phagocytize bacteria and break down devitalized tissue. In addition, important pro-inflammatory cytokines are released from macrophages which include: TFG-beta, B-FGF, TNF-alpha, PDGF, IL-1 and IL-6.1 It should be noted that lymphocytes are among the last cells to infiltrate the wound bed.1 They release IL-2 which help recruit fibroblasts. By removing these impediments, the wound healing process can easily transition into the proliferative and rebuilding phases.
Figure 1: Inflammatory Phase1
PROLIFERATION
The third essential phase of wound healing is the proliferative phase. Fibroblast propagation dominates this wound-healing segment.2 PDGF
that has been released in the preceding phases stimulate fibroblastic chemo-taxis and collagenase production. Fibroblasts also foster the integration
of matrix metalloproteinase’s (MMPs), which facilitate the permeation of these cells within the ECM environment. MMPs remove impaired collagen and other structural proteins while fibroblasts establish a healthy ECM network.1 Several growth factors stimulate the production of new vessels
that are generated by intrinsic cells within the wound bed.1 Vascular endothelial cells, fibroblasts, epidermal cells, and macrophages ontribute to angiogenesis by the production of β-FGF, TGF-β and VEGF.1 The wound surface is covered with new epithelium that is able to restore bacterial barriers and vascular integrity.2 Keratinocytes proliferate, migrate and differentiate during this phase.1, 2
Furthermore, proliferative mechanisms continually promote development of granulation tissue, neo-angiogenesis, matrix deposition/collagen synthesis and epithelialization among others.3
REMODELING
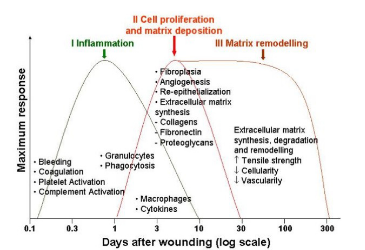
A hallmark feature of the remodeling phase is the maturation of collagen fibers via crosslinking. This promotes increased tensile strength and ibroblast synthesis (Figure 2).1 Collagen fibers steadily condense and in conjunction with myofibroblasts, become oriented parallel to the wound bed along lines of stress, resulting in the appearance of striated scar tissue.1, 2 Contraction of the newly developed ECM could potentially manifest.2 It should be noted the regenerated tissue is not as tensile and dynamic prior to injury.2
Figure 2: Wound Healing Cascade1
BIOCHEMISTRY OF CHRONIC WOUNDS
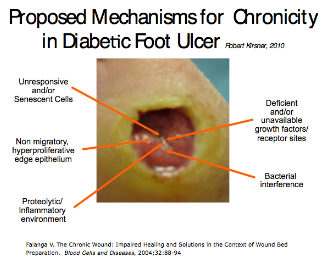
Stalled wounds consist of volatile biochemical mechanisms including increased proteases and inflammatory mediators, unresponsive/senescent cells, hyper-proliferative wound edges (neuropathic ulcerations) and bacterial interference (Figure 4). Nonviable tissue deleteriously effects the wound
environment by fostering bacterial growth and local tissue hypoxia. In order to combat stagnant wound environments, infection and inflammation need to
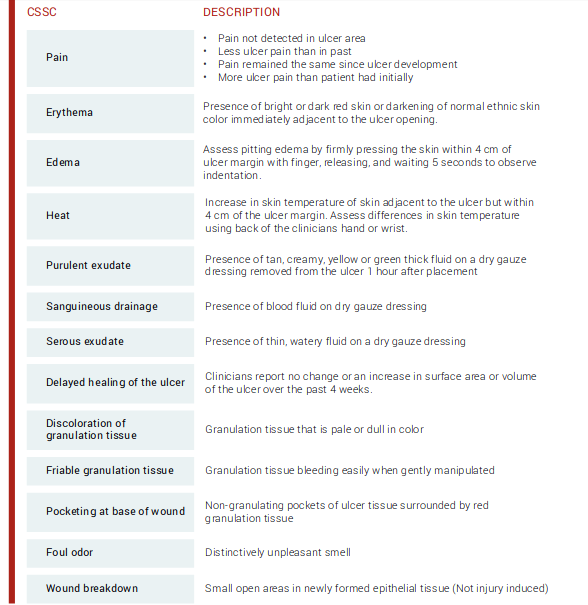
be mitigated. To end these physiologic obstacles, Robson et al discussed the distinctive factors on how bacterial infections and excess granulation tissue impact wound healing.4 They noted that the existence of chronic granulation tissue decreased the amount of antibiotics that reached the wound infection, consequently prolonging the healing process.4 Gardner et al assessed the reliability of clinical tools to evaluate the signs and symptoms of localized infections in chronic wounds.3 Researchers established the “Clinical Signs and Symptoms Checklist,” which centers on primary and secondary signs of infection to be reliable (Figure 3).3 Clinicians should diagnose infection based on the presence of at least two classic symptoms: inflammation or purulent secretions.2 Incorporating a structured methodology to monitor and assess wound infections may improve accuracy in identifying this condition.
Figure 3: Clinical Signs and Symptoms Checklist (CSSC)3
Figure 4: Proposed Mechanism of Chronic Wounds2, 5
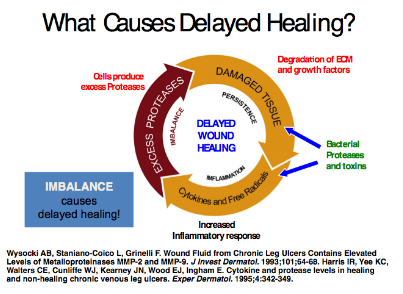
Matrix metalloproteinases (MMP’s) represent a class of 20 protein-degrading enzymes.6 It should be noted that proteases are important during the remodeling phase of wound healing by harmonizing ECM formation. Numerous investigations have revealed that high concentrations of serine protease in neutrophils and elevated MMP’s are responsible for the degradation of ECM proteins, cytokines, growth factors, and cell surface receptors (Figure 5). 7
Figure 5: Excess Protease Activity8, 9
During normal physiological wound healing processes, there is a burst of protease activity at the start of acute wound healing. However, if increased
protease activity is prolonged they have the propensity to degrade the ECM and newly formed tissue.10 In fact, some studies have discovered that increased quantities of specific MMPs (2, 8, 9) and human neutrophil elastase (HNE) are frequently found in chronic wounds in contrast to acute pathologies.10 The deleterious effects of these proteases may stimulate the inflammatory response and discharge harmful reactive oxygen species. There are elevated levels of TNF-α and IL-1β. These molecules induce their own synthesis to set up a cycle of non-progressive inflammation, inhibit collagen formation, and decrease the production of protease inhibitors. It should be noted that the bi-directional communication network between cells and their surrounding ECM are compromised in chronic wound environments.11 This concept is known as dynamic reciprocity (DR), a term first coined by Schultz et al.11 This interaction has been extensively studied and applied to the physiological process of wound
healing. Several therapeutic modalities target this mechanism.11
TREATMENT
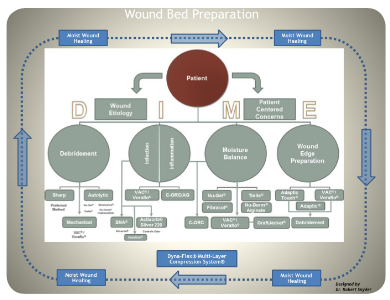
The “DIME” scheme is an all-encompassing modality for proper wound bed preparation.12DIME is a mnemonic that incorporates debridement, infection control, moisture balance, and wound edge preparation (Figure 6).12
Figure 6: DIME & Wound Bed Preparation12
DIME strategy helps accelerate endogenous healing mechanisms by eliminating factors that obstruct the wound-healing sequence. Debridement
treatments are administered to reduce exudate and/or necrotic tissue from the wound. These external modalities include; mechanical, sharp/surgical,
enzymatic debridement, and negative-pressure wound therapy (NPWT) among others.12 Additionally, passive treatments promote the patient’s intrinsic healing mechanisms to help reduce or eliminate impediments to healing.12 Some passive treatments include hydrogels (hydro-polymer dressings), hydrocolloid dressings, autolytic debridement, antimicrobial or antiseptic dressings, collagen-oxidized regenerated cellulose dressings and non-adherent silicone dressings.12
CONCLUSION
Wound management is a specialty that is continuously evolving; wound specialists have a range of treatment options that include biologic skin
substitutes, collagen matrices, negative pressure and active dressings among others. It is vital clinicians routinely base their treatment plans on evidence-based research. Additionally, mastery of wound science will lead clinicians to proper treatment pathways.
References
1. Bryant RA Nix DP. Acute & Chronic Wounds: Current Management Concepts. St Louis, MO: Elsevier/Mosby; 2012.
2. Falanga V. The chronic wound: impaired healing and solutions in the context of wound bed preparation. Blood Cells, Molecules, and Diseases. 2004;32(1):88–94. doi: 10.1016/j.bcmd.2003.09.020.
3. Gardner SE, Frantz RA, Saltzman CL. Diabetes and inflammation in infected chronic wounds. Wounds2005;17(8): 203-205.
4. Robson MC. Wound infection: a failure of wound healing caused by an imbalance of bacteria. Surg Clin North Am. 1997;77(3):637-651.
5. Robert Kirsner MD Phd Personal Communication 2010
6. Armstrong DG, Jude EB. The role of matrix metalloproteinases in wound healing. Journal of the American Podiatric Medical Association. 2002; 92(1): 12-18.
7. Pham, CTN. Neutrophil serine proteases fine-tune the inflammatory response. The International Journal of Biochemistry & Cell Biology. 2008; 40(6-7), 1317–1333. http://doi.org/10.1016/j.biocel.2007.11.008.
8. Wysocki, A. B., Staiano-Coico, L., and Grinnell, F., 1993, Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9, J. Invest. Dermatol. 101:6468.
9. Harris IR, Yee KC, Walters CE, et al. Cytokine and protease levels in healing and non-healing chronic venous leg ulcers. Exp Dermatol. 1995;4:342–349.
10. Gibson D, Cullen B, Legerstee R, et al. MMPs Made Easy. Wounds International 2009; 1(1). http://www. woundsinternational.com.
11. Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen. 2011;19(2):134–148.
12. Snyder RJ, Fife C, Moore Z. Components and Quality Measures of DIME (Devitalized Tissue, Infection/Inflammation, Moisture Balance, and Edge Preparation) in Wound Care.” Advances in Skin & Wound Care 29.5 (2016): 205-215.
References
1. Cowan, Linda, et al. Caution: when combining topical wound treatments, more is not always better. Wound Practice & Research: Journal of the Australian Wound Management Association 19.2 (2011): 60.
2. Armstrong D, Bates-Jensen B, Bohn G, et al. Expert recommendations for the use of hypochlorous solution: science and clinical application. Wounds. 2015.
3. Scanlon, E., and Stubbs, N. To use or not to use? The debate on the use of antiseptics in wound care. British journal of community nusring 7. Sup2 (2002): 8-20.
4. Atiyeh, B. S., Dibo, S. A., & Hayek, S. N. (2009). Wound cleansing, topical antiseptics and wound cleansing, topical antiseptics and wound healing. International Wound Journal, 6(6), 420-430.
5. Drosou A, Falabella A, Kirsner RS. Antiseptics on Wounds: An Area of Controversy. Wounds. 2003; 15(5):149-166.
6. Mulder GD, Cavorsi JP, Lee DK. Antimicrobial Agents in Wound Care. Wounds. 2007;19(7):173-182.
7. McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev.1999; 12(1):147-179.
8. Senior N. Some observations on the formulation and properties of chlorhexidine. J Soc Cosmet Chem. 24(4): 259-278.
9. Basrani BR, Manek S, Sodhi R, Fillery E, Manzur A. Interaction between sodium hypochlorite and chlorhexidine gluconate. J Endod. 2007; 33(8): 966-969.
10. Yasuda K, Ohmizo C, Katsu T. Potassium and tetraphenylphosphonium ion-selective electrodes for monitoring changes in the permeability of bacterial outer and cytoplasmic membranes. J Microbiol Methods. 2003;54(1):111-115.
11. Jovanovic A, Ermis R, Mewaldt RS, Shi L, Carson D. The Influence of Metal Salts, Surfactants, and Wound Care Products on Enzymatic Activity of Collagenase, the Wound Debriding Enzyme. Wounds. 24(9):242–253.
12. Safety and effectiveness of consumer antiseptics; topical antimicrobial drug products for over-the-counter human use; proposed amendment of the tentative final monograph; reopening of administrative record; proposed rule. Fed Regist 78:76444–76478. http://www.gpo.gov/fdsys/pkg/FR-2013-12-17/pdf/2013-29814.pdf
13. Khan M, Naqvi AH. Antiseptics, iodine, povidone iodine and traumatic wound cleansing. J Tissue Viability. 2006; 16(4):6-10.
14. Smith RG. Critical discussion of the use of antiseptics in acute traumatic wounds. J Am Podiatr Med Assoc. 2005; 95(2):148-153.
15. Schmitz G. Iodine oxidation by hydrogen peroxide and Bray–Liebhafsky oscillating reaction: effect of the temperature. Phys Chem Chem Phys. 13(15): 2011: 7102-7111. doi: 10.1039/C1CP00006C
16. Sakarya S, Gunay N, Karakulak M, Ozturk B, Ertugrul B. Hypochlorous Acid: an ideal wound care agent with powerful microbicidal, antibiofilm, and wound healing potency. Wounds. 2014; 26(12):342-350.
17. Thorn RM, Lee SW, Robinson GM, Greenman J, Reynolds DM. Electrochemically activated solutions: evidence for antimicrobial efficacy and applications in healthcare environments. Eur J Clin Microbiol Infect Dis. 2014; 31(5):641–653.
18. Edwards K. New Twist on an Old Favorite: Gentian Violet and Methylene Blue Antibacterial Foams. Adv Wound Care(New Rochelle). 2016; 5(1): 11–18.
19. Hydrofera Blue Classic Antibacterial Foam Dressing; SDS HB2214/HB4414-00 Safety [Online]; Hollister: Libertyville, IL, April 13, 2016. http://www.hollister.com/~/media/files/pdfs%E2%80%93for%E2%80%93download/sds/sds%E2%80%93hydrofera%E2%80%93blue%E2%80%93classic.pdf?la=en (accessed April 15, 2017)
20. Chao C, Runde D. Tap Water vs. Sterile Saline for Wound Irrigation. Am Fam Physician. 2015; 92(3). http://www.thennt.com/nnt/tap-water-for-wound-irrigation/. Accessed February 10, 2017
21. Kim P, Attinger C, Crist B, et al. Negative Pressure Wound Therapy with Instillation: Review of Evidence and Recommendations. Wounds. 2015; 27(12):S2-S19.
22. Wolvos T. Wound Instillation — The Next Step in Negative Pressure Wound Therapy. Lessons Learned from Initial Experiences. Ostomy Wound Manage. 2004; 50(11):56-66.