
Dr. Robert Snyder is Professor and Director of Clinical Research and Fellowship Director in wound care and research at Barry University SPM. He has created and implemented the first post residency Wound Management and Research Fellowship for podiatric medical schools. He is certified in foot and ankle surgery by the American Board of Podiatric Surgery and is also a board certified wound specialist. Dr Snyder is past-president of the Association for the Advancement of Wound Care and past-president of the American Board of Wound Management, the certifying body for Wound Care Specialists. In addition to his doctorate, he holds an MSc in Wound Healing and Tissue Science from Cardiff University. His expertise at Cardiff, Wales, was further acknowledged as he was selected as an Internal Marker for MSc dissertations with an Honorary Title. To constantly expand his knowledge and stay current in all aspects of healthcare, he is completing an MBA in Health Management. Dr. Snyder is a key opinion leader and sought after speaker, lecturing extensively throughout the United States and abroad. He was chosen to develop and teach a wound care course for physicians internationally. Dr. Snyder has published several book chapters and over 150 papers in peer reviewed and trade journals on wound care. He serves on the editorial advisory boards of Ostomy Wound Management, Wounds and Podiatry Management and has recently been appointed as a periodic reviewer for the Lancet and NEJM. He has been a Principal Investigator on more than 50 randomized controlled trials for innovative wound healing modalities and products.

Joey Ead is currently a second year medical student at Barry University School of Podiatric Medicine. Prior to attending medical school, Mr. Ead received his Masters Degree in biomedical science with a concentration in wound healing and tissue regeneration. Mr. Ead has been involved in various research studies and has worked closely with wound care specialist Dr. Robert Snyder DPM, MSc, CWS. modeling systems to anticipate surgical complications.
Snyder and Ead_Current Dialogues in Wound Management_2019_Volume 5_Issue 1
INTRODUCTION
Diabetes Mellitus (DM) represents a groupof metabolic disorders resulting in both glucoseover production, peripheral underutilizationor both ultimately leading to hyperglycemia,glycosuria, and severe cases intermittentketoacidosis. According to the Centers forDisease Control (CDC), DM was the 7th leadingcause of death in 2016. 1 The incidence of DMis predicted to increase by 165% between theyears 2000-2050. 2 According to the WorldHealth Organization (WHO), the global diabeticpopulation has risen from 108 million in 1980to 422 million in 2014. 3
Diabetic foot ulcer (DFU) prevalenceis as high as 25% and it is estimated thatapproximately 40-80% of DFUs becomeinfected. 4 The economic burden of DFUcosts Medicare $9-13 billion/year. 5 A 10-yearprospective study on DFU and mortality, foundthat a history of diabetic ulcerations could bea significant predictor of mortality. 6 Patientswho had a record of DFU’s revealed a 49%increased risk of mortality when compared topatients with diabetes with no DFU’s in theirpast medical history.
DIABETES & DIABETIC FOOT ULCERATIONS
DM is a complex, chronic disorder ofcarbohydrate, fat, and protein metabolism.Acute manifestations are primarily caused bydecreased glucose uptake, increased protein catabolism and lipolysis. Environmental andgenetic risk factors can trigger a cascade ofautoimmune reactions, immunosuppression,and induce metabolic stress.The American Diabetes Association(ADA) classifies DM into the following generalcategories: type 1 & 2, gestational diabetesand other specific types of DM caused by otherfactors (Figure 1). 7 DM is generally diagnosedbased on the following plasma glucose tests;fasting plasma glucose (FPG), 2-h plasmaglucose (2-h PG) value during a 75-g oralglucose tolerance test (OGTT), or A1C criteria(Figure 2).
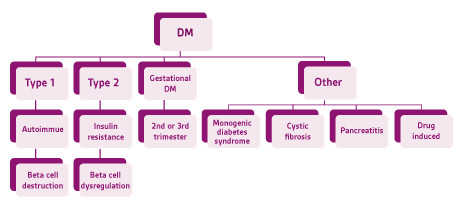 Figure 1. American Diabetes Association diabetes mellitus classification. Adapted from the American Diabetes Association 2017. 7 DM= diabetes mellitus
Figure 1. American Diabetes Association diabetes mellitus classification. Adapted from the American Diabetes Association 2017. 7 DM= diabetes mellitus
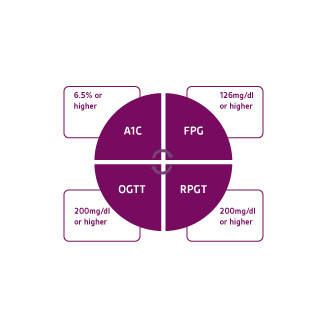 Figure 2. American Diabetes Association criteria for the diagnosis of diabetes mellitus. Adapted from American Diabetes Association 2017. 7 FPG= Fasting plasma glucose; OGTT= Oral glucose tolerance test; RPGT= Random plasma glucose test
Figure 2. American Diabetes Association criteria for the diagnosis of diabetes mellitus. Adapted from American Diabetes Association 2017. 7 FPG= Fasting plasma glucose; OGTT= Oral glucose tolerance test; RPGT= Random plasma glucose test
Normal glucose homeostasis is regulatedbythree biochemical processes: gluconeogenesis,uptake and utilization of glucose by peripheraltissues of the body, and insulin secretion viathe pancreas. The main function of insulin isto increase the rate of transport of glucosefrom the bloodstream into various cells of thebody such as striated muscles. In type 2 DM,metabolic stress can dysregulate Beta-cellfunction and violate the intrinsic homeostaticand hormonal mechanisms that control serumglucose levels causing a permanent stateof hyperglycemia. 7 The liver behaves in agluconeogenic unregulated manner producingexcess glucose. The pancreas responds to thechronic hyperglycemic state with a perpetualinflux of insulin. Ultimately, causing Beta celldysregulation and insulin resistance.
The pathogenesis is more establishedand definitive in type 1 diabetes than in type2 diabetes. 7 It is now evident that immediatefamily members of patients with type 1 DMpresenting with two or more autoantibodies isan almost certain predictor of DM. 7 It shouldbe noted that the rate of progression of type 1DM is dependent on multiple factors; detectionof antibodies, number of antibodies, antibodyspecificity and antibody titer. It should benoted that A1C and glucose levels rise prior tothe clinical onset of DM. 7 Current treatmentmodalities have been insufficient to stopthe progressive trajectory of this metabolicsyndrome and prevent the development of chronic diabetic complications. One potential explanation is that the diagnosis of diabetes is primarily based on measurement of only one metabolite (glucose).
“Pre-diabetics” also pose a major concern. The Centers for Disease Control and Prevention (CDC) estimates approximately 86 million Americans are considered to be pre-diabetic. It should be noted that pre-diabetic patients may not present with clear symptoms. This manifestation is also known as impaired fasting glycaemia, is typically diagnosed when with an A1C of 5.7% – 6.4%, fasting blood glucose of 100 – 125 mg/dl, or an OGTT 2-hour blood glucose of 140 mg/dl – 199 mg/dl. 7 Studies have revealed that there is a 50% risk over 10 years of progressing to type 2 DM. However, many newly identified pre-diabetic patients can progress to DM in less than 3 years. 7;8 Pre-diabetic patients can lower their risk for type 2 DM by 58% by losing 7% of their total body weight and exercising moderately 30 minutes a day, five days a week.
FOOT ULCERS IN PATIENTSWITH DIABETES (DFU): APOTENTIALLY DEVASTATINGCOMPLICATION OF THE DISEASE
Patients with DM and neuropathy are at riskof developing ulcerations. 12 Distal symmetricalpolyneuropathy (DSPN) is the more commonform of DM neuropathy that generally affectsthe toes and distal foot but has the tendency toprogress proximally in a stocking distribution. 12A chronic hyperglycemic state causes sensorynerve hypoxia (within the endoneurium) thusaltering their electrical stability leading tonerve damage. 12 There is increasing evidencethat oxidative stress is triggered by increasedfree radical formation due to impaired glucosemetabolism itself and/or deficits in antioxidantdefense. 13 Patients that suffer with diabeticneuropathic pain (DNP) characterize theirsymptoms as burning, tingling, sharp andshooting sensations. 12 The pain could be amajor culprit to the withdrawal of social andreactional activities and has been associatedwith depression. 12;13
In addition to neuropathy, DFU’s are a form ofchronic wounds that fail to heal due to severalkey factors: ischemia, decreased angiogenicresponse systems, endothelial dysfunction andincreased susceptibility to wound infection. 14Hence, these wounds are classified into three distinct subtypes: neuropathic, ischemic, or neuro-ischemic (Figure 3). Approximately, 50% of DFU’s are neuropathic, 35% are neuro- ischemic and about 15% are ischemic. 15 Having a solid understanding of these variants will help dictate treatment pathways and optimize patient outcomes. Increased blood glucose levels activate a series of events that trigger an accumulation of lipid deposits within the arterial network of large and small vessels. This can lead to stroke, myocardial infarction, and inadequate perfusion to the lower extremity. 16 Peripheral arterial disease (PAD) can predispose these patient populations to sores, skin ulcerations, or gangrene (Figure 4)
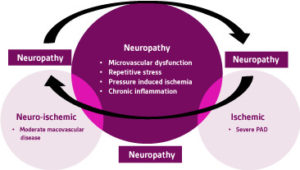 Figure 3. DFU Neuropathy VENN Diagram. DFU = Diabetic Foot Ucler PAD = Peripheral arterial disease
Figure 3. DFU Neuropathy VENN Diagram. DFU = Diabetic Foot Ucler PAD = Peripheral arterial disease
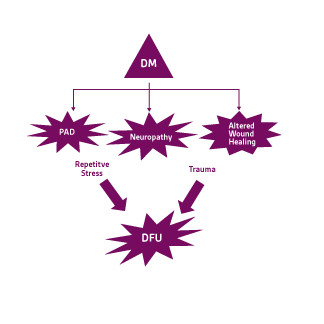 Figure 4. Pathophysiology of diabetic foot ulcers. Adapted from Snyder et al 2010 and Snyder et al 2009 .6;17 DM= diabetes mellitus; PAD=Peripheral arterial disease; DFU= diabetic foot ulcer.
Figure 4. Pathophysiology of diabetic foot ulcers. Adapted from Snyder et al 2010 and Snyder et al 2009 .6;17 DM= diabetes mellitus; PAD=Peripheral arterial disease; DFU= diabetic foot ulcer.
ASSESSMENT AND EVALUATION OF DFUs:
Management of DFU’s is multifacetedand entails a systematic approach for optimalpatient outcomes. For both outpatients andinpatients with a DFU or infection, medicalestablishments should attempt to providea well-coordinated approach by utilizing amultidisciplinary diabetic foot care team. Acomprehensive evaluation for patients withdiabetes encompasses several criteria: the vitalcomponents include: a thorough history andphysical examination, laboratory screening,and a lower extremity focused neurologic,musculoskeletal and vascular assessment. 17Determining the ratio of systolic bloodpressure in the ankle to the systolic bloodpressure in the brachial artery (ABI) operating asphygmomanometer and a hand-held Dopplermachine is a simple, reliable, noninvasive,bedside procedure to assess for peripheralarterial disease. 17 However, there are patientpopulations with diabetes that may displayfalsely elevated ABI’s secondary to medialarterial sclerosis. 17 In this scenario, a toebrachial index (TBI) should be administeredsince the pedal arterial network is not at risk ofmedial arterial sclerosis.
If infection is present, the InfectiousDisease Society of America recommendsobtaining cultures prior to starting empiricantibiotic therapy. 18 Cultures should be takenfrom deep tissue via curettage, utilizationof the Levine Technique or biopsy after thewound has been irrigated and debrided. 18However, samples should only be obtainedif the ulcer appears clinically infected or thepatient is not responding to empiric therapy. 18Infection should always be suspected inany foot wound in a patient with diabetes.However, it should be noted that diabetics andimmunocompromised patients may exhibitonly subtle or no signs of infection. 18 Evidenceof infection generally includes classic signsof inflammation (redness, warmth, swelling,tenderness, or pain) or purulent secretions, butmay also include additional or secondary signs(eg pain in an otherwise painless foot, wounddeterioration, non-purulent secretions, friableor discolored granulation tissue, underminingof wound edges, and/or foul odor). 18 A positiveprobe-to-bone (PTB) test may be a clinicalmarker for osteomyelitis (OM) and should raisesuspicion that this entity exists. 19 Although, bone histopathology and culture studies are the gold standard for the diagnosis of OM, the resources or expertise to perform these invasive procedures are typically unavailable in general medical settings.18 Therefore, many clinicians opt to utilize other diagnostic markers such as the PTB test. Magnetic resonance imaging (MRI) is considered the most effective imaging technique for detecting infections in bone and soft tissue.17 Other tests may include Ceretec or Indium white blood cell scans.17 While a triple phase bone scan lacks specificity, it may be used in conjunction with these test for dual peak imaging.
BIOFILM COMPONENT:
Diabetic foot ulcerations are colonized by numerous bacterial communities. The concomitant factors of patients with diabetes with neuropathy increases the risk of the skin damage promoting bacterial colonization. The frequent bacterial genera that have been associated with the DFU ecosystem remain polymicrobial and commonly include staphylococcus, streptococcus, pseudomonas, Corynebacterium, enterococcus, Acinetobacter, porphyromonas, and other subgroups of the enterobacteriaceae family.20 These deleterious networks have the potential to rapidly progress into deeper tissues causing increased complications.
Primary empiric therapy needs to be implemented based on the severity of the infection and all microbiological data, such as recent culture results and the local prevalence of pathogens, especially antibiotic-resistant strains.20 In recalcitrant lesion’s rapid PCR genetic testing has been the favored over traditional culture techniques.20 Molecular analytic testing has not yet been able to differentiate biofilm from planktonic bacterial species.20 However, molecular studies give the clinician a better understanding of the poly-microbial ecosystem versus standard culture techniques.
The exact pathogenesis of the DFU microbiome is not well understood. However, bacterial biofilm formation seems to play an integral factor in the recalcitrant nature of these wounds. Biofilm consists of a self-generating extracellular matrix (ECM) of robust extra polymeric substances (EPS) that have the ability to irreversibility attach to various parts of the wound bed.20 As these microbial cells begin to multiply and differentiate, their gene expression rapidly evolves in order to promote their survival. This process is known as quorum sensing.20 This phenomenon makes these biofilm communities resistant to host immune responses and increased resistance to antimicrobial therapies. Malone et al determined that the prevalence of biofilm in chronic wounds was 78.2%.21 There is a growing consensus that biofilms play a pivotal role in delaying keratinocyte migration and tissue granulation.20 This has placed a greater emphasis on correctly diagnosing and managing biofilm associated chronic wounds. There is currently no diagnostic modality that helps us understand which biofilms are protective and which are virulent in nature. A biofilm should be clinically suspected when the following symptoms include: thick, tenacious fibrin slough that is non-responsive to sharp debridement or a friable- hyper-granular wound base.
CLASSIFICATION SYSTEMS
The increased value of incorporating a classification guideline in the clinical setting cannot be overstated. Over that past several years, numerous foot-ulcer classification methods have been proposed, however, none of the proposals have been universally accepted. Currently, the two most implemented classification systems include: the Wagner and University of Texas (UT).17 The Wagner system stratifies ulcers primarily on depth and the presence of osteomyelitis or gangrene by using a numeric grading method.17 Vascular disease and the extent infection are not well defined in the Wagner system.17 For instance, superficial wounds that are ischemic or infected cannot be adequately stratified in this classification system.
The UT system improved on the limitations of the Wagner system by grading ulcers by depth and then staging them based on the absence or presence of ischemia and infection.17 They incorporated a grading matrix scored 0 to 3 and scales (scored A to D) to assess ulcer depth along with the presence of lower extremity ischemia and wound infection.17 This system allows clinicians to identify infection and vascular disease as independent factors (despite ulcer depth). However, the UT system fails to stratify the degree of vascular insufficiency.23 A critical question for the specialist is to assess the potential benefit from a successful revascularization procedure which is an essential factor for successful limb preservation.23
Validated classification protocols such as the IDSA or wound, ischemia and foot infection (WIfI) classification systems should be used as a reference point in order to help stratify infections and to help define the severity of concerning cases.23 In 2014, The Society for Vascular Surgery devised a stratification system for threatened lower limbs, grading and categorizing 3 vital risk factors that could potentially lead to amputation.23 The benefit of utilizing the WIfI classification system is that it systematically classifies heterogenous populations with limb-threatening conditions by incorporating many of the preceding guidelines.23 It should be emphasized that when classifications systems are used appropriately, it is a stride forward for quality reporting and patient data aggregation provided the ulcer metrics are consistent.
TREATMENT OF DFUs
Routine management strategies rely heavily on proper wound bed preparation (WBP). By establishing a proper wound environment, advanced products are able to optimize the wound-healing cascade. The DIME model starts by addressing all patient concerns and the underlying comorbidities (Figure 5).24This methodical process calls for extensive WBP in order to accelerate endogenous healing and eliminate any factors that prevent the wound-healing cascade to take its natural course.24 Snyder et al found if a DFU has not reduced by half its percent area reduction the first 4 weeks of care, it is wise to re-evaluate the causes of tissue damage and to consider advanced treatment modalities to avert further complications.
 Figure 5. DIME Treatment options. Adapted from Snyder et al 2016.
Figure 5. DIME Treatment options. Adapted from Snyder et al 2016.
Single strategies used for the treatment of DFU’s may not be effective if biofilm is present. DFU’s could present with delayed epithelialization as a result of decreased cellular proliferative and migratory capacity due to the formation of epiboly’s (rolled in wound edges).20 This can cause defective formation of the supporting stroma. Sharp debridement targets the physical factors such as insidious pressure formation by removing any excess callus or non-viable tissue that may plague wound environment.20 In a retrospective study by Wilcox et al, in more than 300,000 wounds, they found that weekly debridement’s were associated with faster healing times.19;23Frequent, disruptive, and sharp debridement of the wound bed provides a therapeutic window that may lower the biofilm burden. Topical antiseptics include silver, iodine and polyhexamethylene biguanide (PHMB).20;25These topical agents are more effective after debridement by actively penetrating the exopolymeric matrix of biofilm and eradicating the free planktonic bacteria.20 It should be noted that recent studies have revealed that there is less than a 24-hour window in which these therapies are successful.22 A mature biofilm may be present within 72 hours.22 This emphasizes the importance of adhering to a robust and timely biofilm based “step-down” treatment algorithm (Figure 6).
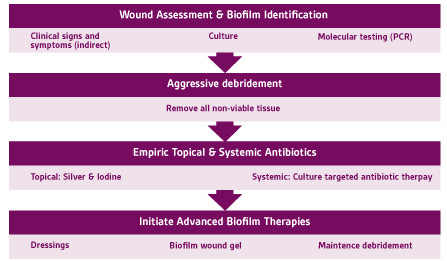 Figure 6. Step down biofilm treatment algorithm. Adapted from Snyder et al 2017.
Figure 6. Step down biofilm treatment algorithm. Adapted from Snyder et al 2017.
Moisture balance is a critical component to improve wound healing.17;24 Excessive exudate formation may lead to maceration which can cause the wound to stagnate.24Therefore, it is paramount to minimize the negative biochemical factors by applying the appropriate dressings based on the wound bed characteristics (size, depth, and moisture levels). It is not recommended to use topical antimicrobials for clinically uninfected wounds. It should be noted that altered biomechanics may predispose to diabetic foot ulcers and impaired wound healing. Depending on the location of the DFU, redistribution of pressure off the wound (off-loading) should be implemented to help reduce the physical factors impeding the wound healing trajectory.17;24 Micropore particle technology (MPPT) is a new treatment modality that does not incorporate antimicrobial activity.26 This new technology exploits the skins microbiome with a dressing that consists of highly porous particles triggering a combination of capillary flow and evaporation to remove exudate.24Bilyayeva et al found MPPT, reduced the time to reaching a wound free of necrosis, pus, and infection by 60%.27 They also found that MPPT promoted a 50% increase rate of granulation and epithelialization; the effects were independent of wound type.26 Subsequent to debridement, silver and Cadexomer iodine based dressings have also shown promising results in the treatment of biofilm infested wounds.27 Over the past couple decades, negative pressure wound therapy (NPWT) has revolutionized the management of complex wounds by its ability to promote granulation tissue formation and help remove infectious materials. With the rise of antimicrobial drug resistance there has been an increased effort investigating alternative treatment modalities. Probiotics, bacteriophages and antimicrobial peptides (AMP) have recently been hypothesized as potential alternatives in the treatment of biofilm infested wounds.
ASSESSMENT AND EVALUATION OF DFUs:
A robust multi-modal and multidisciplinary framework should be adhered for all patients suffering with DFU’s. A timely and accurate diagnosis of a DFU is the first essential step in determining the appropriate treatment pathways. Utilizing evidence-based classification systems can help medical teams stratify the extent of the condition and help dictate treatment protocols Patient centered factors such as diabetes management, nutrition, obesity management, uncontrolled infection, medications, and pain among others can obstruct the wound healing process. Proper wound bed preparation is essential in treating infected DFU’s which involves debridement, managing infection and persistent inflammation, moisture balance, and wound edge optimization. Once the wound has been sufficiently prepared, a precise treatment plan can be determined based on the wounds specific criteria such as: biofilm/bioburden factors, vascularity, extent of infection and other systemic factors. Clinicians should be aware that if there is less than a 50% change in wound size in 4 weeks or not improved in a 2-week time period, advanced therapies should be considered.
References
1.Centers for Disease Control and Prevention. FastStats: Leading Causes of Death. www cdc gov. 2017; https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm. Accessed February 21, 2019.
2.Boyle JP, Honeycutt AA, Narayan KM et al. Projection of diabetes burden through 2050: impact of changing demography and disease prevalence in the U.S. Diabetes Care 2001;24(11):1936-1940.
3.World Health Organization. Global Report on Diabetes. Geneva, Switzerland: World Health Organization; 2016.
4.Richard JL, Sotto A, Lavigne JP. New insights in diabetic foot infection. World Journal of Diabetes 2011;2(2):24-32. doi:10.4239/wjd.v2.i2.24.
5.Geraghty T, LaPorta G. Current health and economic burden of chronic diabetic osteomyelitis. Expert Review of Pharmacoeconomics and Outcomes Research 2019;1-8. doi:10.1080/14737167.2019.1567337.
6.Snyder RJ, Hanft JR. Diabetic foot ulcers – effects on quality of life, costs, and mortality and the role of standard wound care and advanced-care therapies in healing: a review. Ostomy Wound Manage 2009;55(11):28-38.
7.American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care 2017;40(Suppl 1):S11-S24. doi:10.2337/dc17-S005.
8.DeFronzo RA, Abdul-Ghani M. Type 2 diabetes can be prevented with early pharmacological intervention. Diabetes Care 2011;34(Suppl 2):S202-S209. doi:10.2337/dc11-s221.
9. Skyler JS, Bakris GL, Bonifacio E et al. Differentiation of Diabetes by Pathophysiology, Natural History, and Prognosis. Diabetes 2017;66(2):241-255. doi:10.2337/db16-0806.
10.Centers for Disease Control and Prevention. Diabetes Report Card 2014. www cdc gov 2015.
11.Colberg SR, Sigal RJ, Fernhall B et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care 2010;33(12):e147-e167. doi:10.2337/dc10-9990.
12.Bansal V, Kalita J, Misra UK. Diabetic neuropathy. Postgrad Med J 2006;82(964):95-100. doi:10.1136/pgmj.2005.036137.
13.Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World Journal of Diabetes 2015;6(3):456-480. doi:10.4239/wjd.v6.i3.456.
14.Frykberg RG, Banks J. Challenges in the Treatment of Chronic Wounds. Adv Wound Care 2015;4(9):560-582. doi:10.1089/wound.2015.0635.
15.Armstrong DG, Cohen K, Courric S, Bharara M, Marston W. Diabetic foot ulcers and vascular insufficiency: our population has changed, but our methods have not. Journal of Diabetes Science and Technology 2011;5(6):1591-1595.
16.Rask-Madsen C, King GL. Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metabolism 2013;17(1):20-33.
17.Snyder RJ, Kirsner RS, Warriner RA, III, Lavery LA, Hanft JR, Sheehan P. Consensus recommendations on advancing the standard of care for treating neuropathic foot ulcers in patients with diabetes. Ostomy Wound Manage 2010;56(4 Suppl):S1-S24.
18.Lipsky BA, Berendt AR, Cornia PB et al. 2012 infectious diseases society of america clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2012;54(12):e132-e173
19. Grayson ML, Gibbons GW, Balogh K, Levin E, Karchmer AW. Probing to bone in infected pedal ulcers. A clinical sign of underlying osteomyelitis in diabetic patients. J Am Med Assoc 1995;273(9):721-723.
20.Snyder RJ, Bohn G, Hanft J et al. Wound Biofilm: Current Perspectives and Strategies on Biofilm Disruption and Treatments. Wounds 2017;29(6):S1-S17.
21.Malone M, Bjarnsholt T, McBain AJ et al. The prevalence of biofilms in chronic wounds: a systematic review and meta-analysis of published data. J Wound Care 2017;26(1):20-25. doi:10.12968/jowc.2017.26.1.20 rtyp- generic.
22.Wilcox JR, Carter MJ, Covington S. Frequency of debridements and time to heal: a retrospective cohort study of 312 744 wounds. JAMA Dermatology 2013;149(9):1050-1058. doi:10.1001/jamadermatol.2013.4960.
23.Mills JL, Sr., Conte MS, Armstrong DG et al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg 2014;59(1):220-234. doi:10.1016/j.jvs.2013.08.003.
24.Snyder RJ, Fife C, Moore Z. Components and Quality Measures of DIME (Devitalized Tissue, Infection/Inflammation, Moisture Balance, and Edge Preparation) in Wound Care. Adv Skin Wound Care 2016;29(5):205-215. doi:10.1097/01.ASW.0000482354.01988.b4.
25.Phillips PL, Yang Q, Sampson E, Schultz G. Effects of antimicrobial agents on an In Vitro biofilm model of skin wounds. Adv Wound Care 2010;1:299-304.
26.Bilyayeva OO, Neshta VV, Golub AA, Sams-Dodd F. Comparative Clinical Study of the Wound Healing Effects of a Novel Micropore Particle Technology: Effects on Wounds, Venous Leg Ulcers, and Diabetic Foot Ulcers. Wounds 2017;29(8):1-9.
27.Akiyama H, Oono T, Saito M, Iwatsuki K. Assessment of cadexomer iodine against Staphylococcus aureus biofilm in vivo and in vitro using confocal laser scanning microscopy. J Dermatol 2004;31(7):529-534.Santos R, Veiga AS, Tavares L, Castanho M, Oliveira M. Bacterial biofilms in diabetic foot ulcers: potential alternative therapeutics. In: Dhanasekaran D, Thajuddin N, eds. Microbial Biofilms: Importance and Applications. London, UK: IntechOpen Limited; 2016:251-269

