
Stéphanie F. Bernatchez, PhD, is a Senior Research Specialist at 3M. Her work experience includes research and product development in the areas of advanced wound care and skin integrity using in vitro and in vivo assays, as well as clinical research. She received her PhD in Interdisciplinary Sciences from the University of Geneva, Switzerland.

Kimberly Schommer RN, BSN, PHN is currently working as an Clinical Application Specialist, supporting 3M’s healthcare products within the Medical Solutions Division in St. Paul, Minnesota. She is a registered nurse and obtained her Bachelor of Science Degree in Nursing from Winona State University, Winona, MN. She is currently working on obtaining her Master’s in Public Health Degree through the
University of Minnesota. Her previous nursing experience has included pediatric and neonatal intensive care, as well as
coordinating care for children with rare diseases in a pediatric outpatient specialty clinic. This rare disease work was done
in conjunction with the Newborn Screening Program though the state of Minnesota Department of Health.
Kimberly also has both her PHN (Public Health Nursing) and VA-BC certifications.
Bernatchez-Schommer_Current-Dialogues-in-Wound-Management_2021_Article 15
Healthcare-associated infections (HAIs) are largely preventable. These infections are linked to a variety of risk factors such as indwelling medical devices, surgical procedures, injections, contamination of various surfaces in the environment, and exposure to communicable diseases from other patients and healthcare professionals. Working to reduce and eventually eliminate them will save lives and reduce costs.
The Centers for Disease Control and Prevention (CDC)’s National Healthcare Safety Network (NHSN) has been collecting data for several years on specific HAIs.1 Progress has been made over time in preventing central line-associated bloodstream infections (CLABSI), catheter-associated urinary tract infections (CAUTIs), Surgical Site Infections (SSIs), Methicillin-resistant Staphylococcus aureus (MRSA) bacteremia, and Clostridium difficile infections (CDIs). These infections are relatively straightforward since they can be associated with specific devices, procedures, or pathogens. In addition to the major causes listed above, other HAIs may occur due to accidental transmission of an infectious agent through a contaminated surface. Establishing causality and tracking these infections can be more challenging; nevertheless, clinicians are gaining awareness of the fact that microbial transmission can occur through a variety of activities in the care setting.2 Hooker et al3 have followed the trajectory and touch points of various objects such as trolleys, gloves, curtains, surgical tape, and many other items, and report that the complexity of practice, rather than compliance failure, often contributes to potential microbe transmission. The COVID-19 pandemic brings a whole new level of concern to these undesirable occurrences and greater scrutiny of current practices is directed at eliminating these potentially avoidable contributions to infection transmission.
Medical tape is one of the most routinely used items in the healthcare environment, with more touchpoints during its life cycle than any other medical device. While the evidence surrounding its role in the risk of cross contamination has been reported on for decades, the lack of formal guidance around its use and storage highlights the lack of recognition for its important role in providing safe and effective care to patients. The standard precautions for all patient care recommended in the current guidelines from the CDC state that low-level disinfection should be performed for noncritical patient-care surfaces and equipment that touch intact skin, and that noncritical patient-care devices should be disinfected on a regular basis (based on the Spaulding classification).4 Since tape is considered noncritical AND it cannot be disinfected, by inference from these guidelines, a new single use tape roll should be used on each patient and then discarded. However, tape is sometimes used near non-intact skin, such as intravascular (IV) access sites. We reviewed the published medical literature to present the evidence available on the potential of medical tape to be a vector of infection transmission and to propose recommendations.
MATERIAL AND METHODS
A literature search was conducted for cross-contamination due to medical tape use in 6 databases through STNext (Medline, Embase, Biosis, Toxcenter, Chemical Abstracts and PQScitech) in June 2020. The search strategy used database specific indexing terms for surgical tape, adhesive agent, adhesives or the keyword for tape. This concept was limited to database specific indexing terms for cross-infection or keywords for cross-infection, bacterial transmission or nosocomial. Fifty-one results were identified after 47 duplicates were removed. We did not limit by date and only included articles in English in this review. Additional articles were identified from the bibliographies, as well as one article found in a trade journal.
RESULTS
The literature search resulted in the identification of 42 relevant articles (38 peer-reviewed articles, 1 trade journal article, 2 abstracts, and 1 letter to the editor). Information from the relevant records has been organized by study type and is summarized below (in the text and in Table 1).
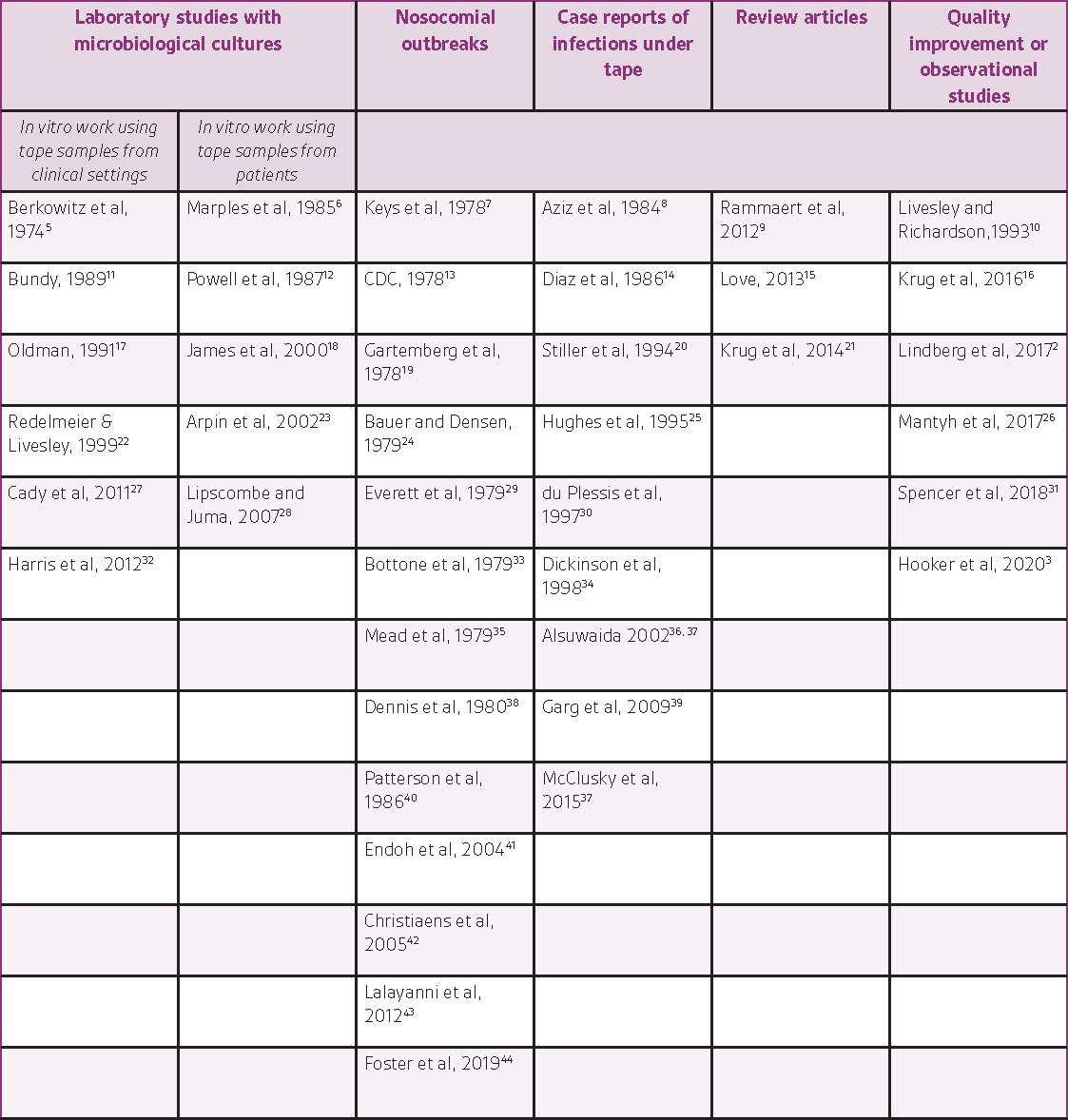
STUDIES WITH MICROBIOLOGICAL CULTURES
Several studies reported in vitro work using tape samples from clinical settings. In a variety of experimental designs, researchers cultured rolls of tape coming out of the manufacturer’s box and at various time points after their release to the supply room,5, 11, 32 after various handling steps (with sterile gloves or bare hands, scissors wiped with alcohol or not, etc.),17, 27 or using rolls already open found around the hospital, e.g. from IV equipment baskets, desktop surfaces in wards, or by asking someone to lend a roll of tape.22 These studies found increasing bacterial contamination of the tapes over time and with additional handling steps and reported the identification of a variety of different organisms: S. epidermidis, Bacillus sp., Klebsiella, S. marcescens, E. coli, P. aeruginosa, S. aureus, M. polymorpha, P. vulgaris, P. mirabilis, fungus, DNase-negative Staphylococci, gram-negative cocci, coagulase-negative and coagulase-positive Staphylococci, alpha hemolytic Streptococcus, Micrococcus, Diphtheroids, Viridans streptococcus, Fusarium, Bipolaris, methicillin-resistant S. aureus (MRSA), vancomycin-resistant Enterococcus (VRE). These microbiological studies have repeatedly demonstrated that rolls of tape can harbor pathogenic bacteria and are therefore cause for concern as they may contribute to infection.
Other in vitro studies used samples from patients. Studies from neonatal intensive care units showed bacterial growth under cardiac monitors and under occlusive plastic adhesive tape,6 and a correlation between the number of days of tape use and colonization.12 Another study18 described a molecular analysis method to type various isolates of Aspergillus flavus and determined that a cluster of infections were related (two cases from a NICU, where both infants were transported by the same ambulance and crew on the same day, and a 4-year old child treated at the same medical center during the same week). Genotyping methods were also used to illustrate how cross-contamination led to a cluster of 4 cases of B. fragilis bacteremia and to demonstrate that this strain remained viable on the adhesive tape for at least 8 hours when present at levels higher than 106 cfu/ml.23
NOSOCOMIAL OUTBREAKS
Several publications starting in the 1970s reported nosocomial outbreaks or case reports of Rhizopus infections associated with Elastoplast products.
7, 13, 19, 24, 29, 33, 35, 38, 40, 42 Two of these publications report fatal outcomes (one patient each).19, 40 Investigations by the FDA and the manufacturer later confirmed Rhizopus species in culture of the product and in environmental samples taken in the plant.7 Although the microorganism did not originate in the hospital in these cases, these articles confirm that contaminated adhesive products such as tapes and dressings can transmit infection to patients. Rammaert9 published in 2012 a review of all the cases of mucormycosis attributed to healthcare procedures between 1970 and 2008. A total of 169 cases were studied. Skin was the most common location (57%). Rhizopus was the most frequent genus (43%). Infection portal of entry included surgery and presence of medical devices such as catheters and drains, adhesive tapes and bandages, and intravascular devices. It is noteworthy that the reported overall mortality rate for this infection is 50%, emphasizing the importance of prevention. Most cases occurred in very sick patients but also occasionally in patients with no predisposing conditions.35
CASE REPORTS
The literature search also identified several case reports of bacterial or fungal infections under tapes used to secure various devices. Each report may have been perceived as an anecdotal description of rare cases at the time of publication, but taken together, they illustrate the growing evidence over time that serious infections can occur due to devices typically seen as innocuous. Necrotic lesions due to mucormycosis were noted under adhesive tape holding endotracheal tubes.8, 34, 36 Phycomycosis (fungus infection of the orbit) was reported in two patients, with the primary lesion located under the adhesive tape used to fix their nasogastric tube to the skin.14 Another fungal infection (cutaneous zygomycosis) was reported under the tape used to secure a nasogastric tube; the authors describe zygomycosis as a “rapidly progressive infection which may be fatal in a few days if not treated”.
In addition, case reports commenting on infections suspected to be related to tapes (but where tapes were not cultured, or indirect or inconclusive results were obtained) have been published.20, 25, 41, 43, 44 For example, a premature infant developed an infection at an abrasion on the abdominal wall “most likely caused by removal of an adhesive patch used to cover the temperature probe”, which developed into fatal zygomycotic necrotizing cellulitis.30 The gravity of this case highlights the importance of this potential risk and the possible consequences in fragile, compromised patients.
DISCUSSION
EVIDENCE
Our literature review indicates that medical tape has been a suspected vector of infection transmission for decades and yet the practice has not changed substantially. As described by Redelmeier in 1999,22 adhesive tape is a unique piece of medical equipment for several reasons: it is not washed or sterilized after initial opening; a given roll may be used by several clinicians on multiple patients; it is frequently manipulated using ungloved hands; and it can be applied in close contact to intravascular insertion sites. In addition, tape can strip skin when repeated applications and removals are needed, further increasing the risk of infection since the skin barrier protection is damaged. Patients who are at increased risk for infection due to their health condition(s) are particularly vulnerable.
The practice around tape started changing with the Centers for Medicare & Medicaid Services guidance for hemodialysis patients issued in 2008,45 stating that “Tape rolls must be dedicated to a single patient, or disposed of after patient use” (Federal Register Vol 73, No 73, page 20376, middle column).
Hemodialysis patients are recognized as being immunosuppressed and thus have a higher susceptibility to infections in general. They are also at increased risk of more severe infections. It would be reasonable to apply similar infection control precautions to all patients on whom tape is used.
Infection risk from tapes has been identified in the literature around other specific procedures. One of them is the securement of endotracheal tubes, often involving cutting non-sterile adhesive tape and adhering it to the anesthesia gas machine before using it for securement on skin.21 The same authors later published a study showing that a change in practice can happen when the best and most current evidence is presented.16<-sup> Another example is the use of tape to remove surgical site hair after clipping.26, 31 This illustrates an example where tape is creatively used for a purpose other than for what it is intended and can once again become a vector for infection if it collected environmental microorganisms prior to its use. Finally, a few articles were identified in which tape was used as a collection device to sample microorganisms from various environments suspected to be contaminated.46, 47 This last example does not constitute a risk of contamination for patients but confirms that microorganisms do adhere and survive on tape.
CLINICAL IMPLICATIONS AND PROPOSED SOLUTIONS
There are currently no specific guidelines regarding the storage and use of tape. Tape often makes its way through the care setting via clinician pockets or on stethoscopes and is stored in areas that are not routinely cleaned. The tape is then subsequently used in the treatment of multiple patients. Increased demand for single-patient use products has emerged as central to infection control practices, however tape continues to be one of the only items still used on multiple patients. As previously reported by McClusky et al,37 a survey completed at a 2014 Michigan Society for Infection Prevention and Control Spring Conference noted 64% of clinicians do not dedicate rolls of tape to a specific patient, nor do they discard used tape rolls when a patient is discharged 57% of the time. Those multi-use rolls of tape are then taken from room to room where they can serve as a vector for transmission between compromised patients. Unsurprisingly, 100% of clinicians surveyed reported that their institution has no policy or standard of care for how tape is stored.
Specific guidance regarding medical tapes should be included in the next updates to existing guidelines on infection prevention. In the interim, healthcare facilities can improve practice by taking the following simple actions and formalizing them in their standard policies and procedures:
Tape rolls should be individually packaged to help reduce potential exposure to environmental contaminants, facility surfaces and equipment, as well as the hands of healthcare professionals;
All tape rolls, regardless of length, should be individually packaged for single use on a single patient;
Unpackaged tape should not be kept in pockets or on stethoscopes;
Tape should be stored in a clean storage or utility room with established cleaning schedules and in the manufacturer’s packaging;
Tape found unpackaged is potentially contaminated and should be disposed of. Conversely, the presence of intact packaging confirms that a new roll is being used.
With the availability of individually packaged single-use length medical tapes, implementation of these recommendations can be easy and straightforward.
CONCLUSIONS
Medical tape is ubiquitous and widely used in the health care setting due to its utility in performing a variety of clinical tasks. Many published case reports point out its role in infectious disease transmission, enabled by storage, handling, and usage practices. Observed practices include carrying tape in clinician pockets and on stethoscopes, storing unused portions in areas that are often not routinely cleaned, and using the same roll in the treatment of multiple patients. Comprehensive clinical practice guidelines with recommendations on medical tape storage, handling, use and application are needed to reduce cross-contamination and HAIs. The emergence of new pathogens such as SARS-CoV-2 brings a new urgency to this topic. The solutions proposed in this article to improve handling of one of the most widely used items in healthcare should be given due consideration.
Note: this is a summarized version of an article published in the American Journal of Infection Control.
Originally published as:
Bernatchez SF, Schommer K. Infection prevention practices and the use of medical tapes. American Journal of Infection Control 49:1177-1182, 2021. DOI: 10.1016/j.ajic.2021.03.007. Available online at Infection prevention practices and the use of medical tapes (ajicjournal.org).
© 2021 3M. All rights reserved. 3M and the other marks shown are marks and/or registered marks. Unauthorized use prohibited. 3M Marks used under license in Canada. All other marks are property of their respective owners.
References
References:
1. CDC. Data Summary of HAIs in the US: Assessing Progress 2006-2016. https://www.cdc.gov/hai/surveillance/data-reports/data-summary-assessing-progress.html, accessed 13 Aug 2020. 2018.
2. Lindberg M, Lindberg M, Skytt B. Risk behaviours for organism transmission in health care delivery-A two month unstructured observational study. International journal of nursing studies. 2017;70:38-45.
3. Hooker C, Hor S, Wyer M, Gilbert GL, Jorm C, Iedema R. Trajectories of hospital infection control: Using non-representational theory to understand and improve infection prevention and control. Soc Sci Med. 2020;256:113023.
4. CDC, Rutala WA, Weber DJ, Healthcare Infection Control Practices Advisory C. Guideline for Disinfection and Sterilization in Healthcare Facilities 2008, Updated May 2019 (accessed 13 Aug 2020). https://wwwcdcgov/infectioncontrol/guidelines/disinfection/index. 2019.
5. Berkowitz DM, Lee WS, Pazin GJ, Yee RB, Ho M. Adhesive tape: Potential source of nosocomial bacteria. Applied Microbiology. 1974;28:651-4.
6. Marples RR, Richardson JF, Seal DV, Cooke EM. Adhesive tapes in the special care baby unit. Journal of Hospital Infection. 1985;6:398-405.
7. Keys TF, Haldorson AM, Rhodes KH, Roberts GD, Fifer EZ. Nosocomial outbreak of Rhizopus infections associated with Elastoplast wound dressings – Minnesota. Morbidity and Mortality Weekly Report. 1978;27:33-4.
8. Aziz S, Merrell RC, Edwards MF. Mucormycosis in patients with multiple organ failure. Archives of Surgery. 1984;119:1189-91.
9. Rammaert B, Lanternier F, Zahar JR, Dannaoui E, Bougnoux ME, Lecuit M, et al. Healthcare-associated mucormycosis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;54 Suppl 1:S44-54.
10. Livesley J, Richardson S. Securing methods for peripheral cannulae. Nursing standard. 1993;7:31-4.
11. Bundy AT. Sterility in unsterilized surgical adhesive tape. Plastic and Reconstructive Surgery. 1989;83:880-3.
12. Powell DA, Hayes J, Durrell DE, Miller M, Marcon MJ. Malassezia furfur skin colonization of infants hospitalized in intensive care units. Journal of Pediatrics. 1987;111:217-20.
13. CDC. Follow-up on Rhizopus infections associated with Elastoplast bandages – United States. Morbidity and Mortality Weekly Report. 1978;27:243-4.
14. Diaz EG, Gutierrez EM, Diaz AG. Fungus infection of the orbit. Orbit. 1986;5:141-4.
15. Love KL. Single-patient rolls of medical tapes reduce cross-contamination risk. Infection Control Today. 2013;17:45-7.
16. Krug L, Machan MD, Villalba J. Changing endotracheal tube taping practice: An evidence-based practice project. AANA J. 2016;84:261-70.
17. Oldman P. A sticky situation? Microbiological study of adhesive tape used to secure IV cannulae. Professional Nurse. 1991;6:265-9
18. James MJ, Lasker BA, McNeil MM, Shelton M, Warnock DW, Reiss E. Use of a repetitive DNA probe to type clinical and environmental isolates of Aspergillus flavus from a cluster of cutaneous infections in a neonatal intensive care unit. Journal of Clinical Microbiology. 2000;38:3612-8.
19. Gartemberg G, Bottone EJ, Keusch GT, Weitzman I. Hospital-acquired mucormycosis (Rhizopus rhizopodiformis) of skin and subcutaneous tissue. Epidemiology, mycology, and treatment. New England Journal of Medicine. 1978;299:1115-8.
20. Stiller MJ, Teperman L, Rosenthal SA, Riordan A, Potter J, Shupack JL, et al. Primary cutaneous infection by Aspergillus ustus in a 62-year-old liver transplant recipient. J Am Acad Dermatol. 1994;31:344-7.
21. Krug L, Machan M, Villalba J. Securing the endotracheal tube with adhesive tape: An integrative literature review. AANA J. 2014;82:457-64.
22. Redelmeier DA, Livesley NJ. Adhesive tape and intravascular-catheter-associated infections. Journal of General Internal Medicine. 1999;14:373-5.
23. Arpin C, Dubois V, Rogues AM, Menard F, Gavinet AM, Maire JP, et al. Cross-infection due to imipenem-resistant Bacteroides fragilis associated with a totally implantable venous port. J Clin Microbiol. 2002;40:3032-4.
24. Bauer E, Densen P. Infections from contaminated Elastoplast. New England Journal of Medicine. 1979;300:370.
25. Hughes C, Driver SJ, Alexander KA. Successful treatment of abdominal wall Rhizopus necrotizing cellulitis in a preterm infant. The Pediatric Infectious Disease Journal. 1995;14:336.
26. Mantyh CR, Xi H, Pearson L, Perl TM. Minimizing hair dispersal: Is this an opportunity for improvement in health care-acquired infection prevention? American journal of infection control. 2017;45:308-10.
27. Cady M, Gross L, Lee N. Letter to the Editor: I.V. Tape: A potential vector for infection. APSF Newsletter. 2011;Winter 2011:61-2.
28. Lipscombe S, Juma A. Bacterial growth on adhesive dressing tapes used for the closure of surgical wounds. European Journal of Plastic Surgery. 2007;29:217-20.
29. Everett ED, Pearson S, Rogers W. Rhizopus surgical wound infection associated with elasticized adhesive tape dressings. Archives of Surgery. 1979;114:738-9.
30. du Plessis PJ, Wentzel LF, Delport SD, van Damme E. Zygomycotic necrotizing cellulitis in a premature infant. Dermatology. 1997;195:179-81.
31. Spencer M, Barnden M, Johnson HB, Fauerbach LL, Graham D, Edmiston CE, Jr. Perioperative hair removal: A review of best practice and a practice improvement opportunity. J Perioper Pract. 2018;28:159-66.
32. Harris PNA, Ashhurst-Smith C, Berenger SJ, Shoobert A, Ferguson JK. Adhesive tape in the health care setting: Another high-risk fomite? MJA. 2012;196:34-.
33. Bottone EJ, Weitzman I, Hanna BA. Rhizopus rhizopodiformis: Emerging etiological agent of mucormycosis. Journal of Clinical Microbiology. 1979;9:530-7.
34. Dickinson M, Kalayanamit T, Yang CA, Pomper GJ, Franco-Webb C, Rodman D. Cutaneous zygomycosis (mucormycosis) complicating endotracheal intubation: diagnosis and successful treatment. Chest. 1998;114:340-2.
35. Mead JH, Lupton GP, Dillavou CL, Odom RB. Cutaneous Rhizopus infection. Occurrence as a postoperative complication associated with an elasticized adhesive dressing. JAMA. 1979;242:272-4.
36. Alsuwaida K. Primary cutaneous mucormycosis complicating the use of adhesive tape to secure the endotracheal tube. Canadian Journal of Anesthesia. 2002;49:880-2.
37. McClusky J, Davis M, Dahl K. A gap in patient tape storage and use practices puts patients at risk for cutaneous fungal infections. American journal of infection control. 2015;43:182-4.
38. Dennis JE, Rhodes KH, Cooney DR, Roberts GD. Nosocomial Rhizopus infection (zygomycosis) in children. The Journal of Pediatrics. 1980;96:824-8.
39. Garg J, Sujatha S, Garg A, Parija SC. Nosocomial cutaneous zygomycosis in a patient with diabetic ketoacidosis. Int J Infect Dis. 2009;13:e508-10.
40. Patterson JE, Barden GE, Bia FJ. Hospital-acquired gangrenous mucormycosis. The Yale Journal of Biology and Medicine. 1986;59:453-9.
41. Endoh M, Okuno R, Mukaigawa J, Shimojima Y, Murata I, Morozumi S, et al. Two nosocomial outbreaks of sepsis caused by Serratia marcescens, which occured in July 1999 and January 2002 – Tokyo. Journal of the Japanese Association for Infectious Diseases. 2004;78:295-304.
42. Christiaens G, Hayette MP, Jacquemin D, Melin P, Mutsers J, De Mol P. An outbreak of Absidia corymbifera infection associated with bandage contamination in a burns unit. The Journal of hospital infection. 2005;61:88.
43. Lalayanni C, Baliakas P, Xochelli A, Apostolou C, Arabatzis M, Velegraki A, et al. Outbreak of cutaneous zygomycosis associated with the use of adhesive tape in haematology patients. The Journal of hospital infection. 2012;81:213-5.
44. Foster C, Revell P, Campbell JR, Marquez L. Healthcare-associated pediatric cutaneous mucormycosis at Texas Children’s Hospital, 2012-2019 (Abstract #2465). Open Forum Infectious Diseases. 2019;6:S853.
45. CMS. 42 CFR Parts 405, 410, 413 et al. Medicare and Medicaid Programs; Conditions for Coverage for End-Stage Renal Disease Facilities; Final Rule. Federal Register https://wwwcmsgov/Regulations-and-Guidance/Legislation/CFCsAndCoPs/downloads/ESRDfinalrule0415pdf (accessed 11 Mar 2021). 2008;73:20370-483.
46. Takuma T, Okada K, Yamagata A, Shimono N, Niki Y. Mold colonization of fiberglass insulation of the air distribution system: effects on patients with hematological malignancies. Med Mycol. 2011;49:150-6.
47. Ogai K, Nagase S, Mukai K, Iuchi T, Mori Y, Matsue M, et al. A Comparison of Techniques for Collecting Skin Microbiome Samples: Swabbing Versus Tape-Stripping. Frontiers in microbiology. 2018;9:2362.

