Lydia Galarza, RN received her nursing degree from the University of Texas at El Paso. She has spent the last 17 years in the medical device industry, with KCI, providing guidance for wound healing and product safety. Ms. Galarza currently serves as the Director for the Global Safety department.

Dr. John D. Short has worked as a medical writer in the medical device industry for approximately two and a half years, where he has assisted healthcare professionals with posters and publications related to wound care, and he has prepared various types of documents related to the execution of clinical trials. He currently works for KCI in San Antonio, TX. Before joining the medical device industry, Dr. Short received his postdoctoral training at the University of Texas M.D. Anderson Cancer Center and the University of Texas Health Science Center at San Antonio, where he subsequently served as a research faculty member. Dr. Short has published numerous research articles pertaining to immunology, cardiovascular disease, obesity and diabetes, and cancer biology. He holds a Ph.D. in Cell Biology and Biochemistry from Texas Tech University Health Sciences Center in Lubbock, TX.
Galarza/Short_Current-Dialogues-in-Wound-Management_2019_Article_6
Historically, V.A.C.® Therapy has used a drape containing acrylic adhesive (V.A.C.® Drape) to cover foam dressings in the wound bed. To offer an alternative to the existing acrylic drape, an innovative film comprised of silicone and acrylic adhesives (V.A.C. DERMATAC™ Drape; Figure 1) has been developed with ease-of-use and patient comfort in mind. A user evaluation was performed for V.A.C. DERMATAC™ Drape to assess for ease of application, seal performance with V.A.C.® Therapy, skin integrity, and patient comfort.

Drape applications using V.A.C.® Therapy at -125 mmHg were administered on 7 female and 10 male patients (n=17) in Chile at 2 hospitals (Hospital San Juan de Dios and Hospital Sotero Del Rio) over a 2-week period during April of 2018 (Table 1). Wound types included diabetic foot ulcers, pressure ulcers, and abdominal wounds (Table 1).
Drapes were applied in one case on a wound that had been covered with a split-thickness graft. A total of 53 drape applications were performed (Table 2), and drapes were changed every 48-72 hours.
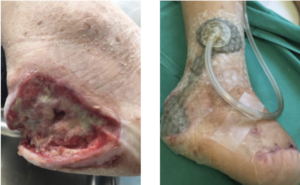
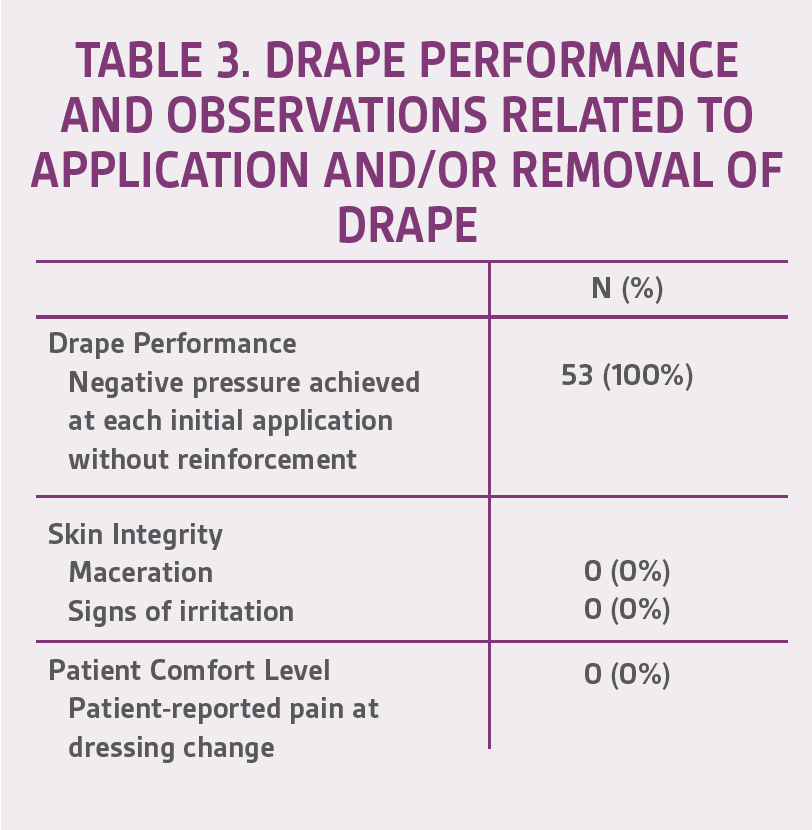
The mean age of patients in this user evaluation was 52.5 ± 17 years. Negative pressure was achieved 100% of the time upon application at each dressing change. During the first week, 15 of 24 (62.5%) drapes maintained a seal for 48 hours without reinforcement; however, after further training of healthcare professionals (HCPs), this percentage increased to 25/29 (86.2%) during week 2. There were no signs of maceration or skin irritation reported, and 100% of the patients claimed no pain upon drape removal based on verbal feedback (Table 3). Representative cases are shown in Figures 2-4.
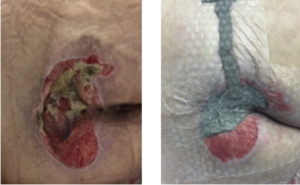
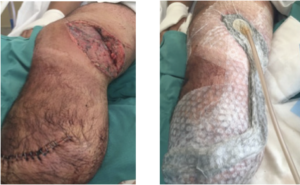
CONCLUSIONS
Data from this initial evaluation suggest that the use of V.A.C. DERMATAC™ Drape provides adhesion to maintain a seal yet is gentle upon removal during dressing changes, thereby potentially improving the HCP and patient experience when undergoing NPWT. The ability to lift and reapply the drape during the initial placement may also help facilitate application by HCPs.
References
NOTE: As with any case study, the results and outcomes should not be interpreted as a guarantee or warranty of similar results. Individual results may vary depending on the patient’s circumstances and condition. Specific indications, contraindications, warnings, precautions and safety information exist for KCI products and therapies. Please consult a clinician and production instructions for use prior to applications. This material is intended for healthcare professionals.

