Dr. Robert Snyder is a podiatrist with over 30 years of experience; his practice is limited to wound management and limb preservation. He is Professor and Director of Clinical Research and Fellowship Director at Barry University SPM. Dr. Snyder is certified in foot and ankle surgery by the American Board of Podiatric Surgery and is also a board certified wound specialist. Dr. Snyder is immediate past-president of the Association for the Advancement of Wound Care and past-president of the American Board of Wound Management. In addition to his doctorate, he holds an MSc in Wound Healing and Tissue Repair from Cardiff University. Dr. Snyder has published several book chapters over 125 papers in peer reviewed and trade journals on wound care and has been a principal investigator on more than 30 randomized controlled trials.
Snyder_Current Dialogues in Wound Management_2015_Volume 1_Issue 2
CHRONIC/STALLED ulcers are often accompanied by underlying comorbidities including diabetes, infection, and peripheral vascular disease, among others. These complex wounds are linked to an aberrant biochemistry resulting in chronic inflammation. Therefore, diagnosis and treatment requires a comprehensive multidisciplinary approach predicated on evidence-based protocols.
The current literature proffers many validated clinical practice guidelines; however, several are convoluted and complex, leading to poor compliance on the part of clinicians. One pragmatic approach involves a simple yet powerful algorithm that prompts specialists and generalists to utilize a systematic approach to wound management; the wound bed preparation (WBP) model. This methodology involves a complete “head-to-toe” evaluation of the patient fostering review of patient-centered concerns and the formulation of potential etiologies. A holistic approach culminates in a “user-friendly road map” to treating the wound, elucidating essential precepts with the acronym DIME:
Debridement
Control of infection/ inflammation
Moisture balance/ imbalance
Wound edge preparation
Debridement3 represents a central tenet of wound management and functions by removing necrotic tissues and slough that harbor bacteria, enzymes, and biofilm. The goal is to rebalance toxic wound biochemistry and create a paradigm shift by converting a chronic wound to one that is acute. This “jump starts” the ulcer and moves the wound environment into the proliferative phase of wound healing, thus fostering a normal healing cascade.
Sharp debridement is usually preferred, if feasible. However, additional methods may also be useful including autolytic, mechanical, enzymatic, and biosurgery methods.
Control of infection4 and inflammation5remains pivotal in re-establishing an appropriate healing trajectory. However, in the immunocompromised patient (e.g., diabetes, autoimmune disease), determining whether there is truly infection may be challenging, as a normal physiologic response (i.e., heat, pain, redness, swelling) may be absent. Often, secondary signs and symptoms (e.g., wound deterioration, pain in an otherwise painless foot) may offer clues, as well as location and lack of response to validated treatment protocols.
Therefore, one can readily see that clinicians may tend to undertreat infections and overtreat inflammation. Cultures may not be indicated unless infection is suspected or when an antibiotic sensitivity pathway is required.4If inflammation is suspected, intervention may consist of topical oxidized regenerated cellulose/collagen to control enzyme activity.
If local infection is likely, agents such as antiseptics (e.g., silver, iodine) may be useful along with systemic antibiotics, if required. However, research by Robson7 hypothesized that systemic antibiotics may not penetrate chronic granulation tissue; this makes a strong case for utilization of topical agents as first-line therapy, absent frank infection. Negative pressure wound therapy (NPWT) with instillation and dwell time has also been shown to have significant benefit in lowering bioburden.
The literature discouraged prolonged use of topical antibiotics as they tend to quickly develop resistance and may sensitize patients to potent agents they may require in the future for more serious infections.4 Antiseptics are favored due to their low resistance potential and broad spectrum of activity.
Moisture balance is critical to support wound healing; wounds that are too wet (i.e., maceration) or too dry (i.e., necrosis) must be addressed to prevent infection and foster the movement of keratinocytes. Treatment is based upon clinical evaluation. For example, maceration may be treated with calcium alginate dressings, collagen matrix dressings, and foams; dry lesions may respond to hydrogels, among others.
Many chronic wounds possess hyperproliferative wound edges, often accompanied with undermining or tunneling. It is imperative to remove this tissue and remodel the wound edges to prevent margination of keratinocytes, foster movement of these essential cells, and prevent epiboly. This is normally facilitated by sharp dissection if feasible.
When bone or tendon is exposed, a thick layer of natural tissue or multiple layers of adherent dressing, if natural tissue is not available, should be used to protect these structures from foam dressings, if using NPWT. An interface between the NPWT foam and the wound (e.g., non-adherent dressings) may prevent granulation tissue from integrating into the foam and help mediate pain on dressing removal. Recent evidence by Lessing et al revealed that granulation tissue may also be substantially augmented with NPWT with instillation and dwell time.
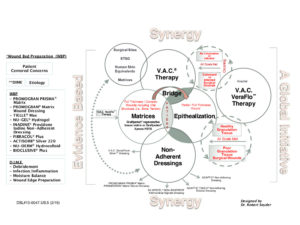
Once granulation tissue is prevalent and wound bed preparation successful, coverage with split-thickness skin grafts, flaps, or epidermal blister grafts may be the next logical steps in the reconstruction ladder. Using therapies sequentially or combining them in a logical approach (with WBP as the centerpiece) may lead to synergism. (Figure 1)
References
1. Sibbald RG, Orsted HL, Coutts PM, et al. Best practice recommendations for preparing the wound bed: update 2006. Adv Skin Wound Care. 2007;1;20(7):390-405.
2.Schultz G, Mozingo D, Romanelli M et al. Wound healing and TIME; new concepts and scientific applications. Wound Repair Regen. 2005;13(4 Suppl):S1-S11.
3.Snyder R, Kirsner RS, Warriner RA 3rd, et al. Consensus recommendations on advancing the standard of care for treating neuropathic foot ulcers in patients with diabetes. Ostomy Wound Manage. 2010;56(4 Suppl):S1-S24.
4.Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Disease Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54(12):e132-73.
5.Snyder, Cullen B, Nisbet LT. An audit to assess the perspectives of US wound care specialists regarding the importance of proteases in wound healing and wound assessment. Int Wound J. 2013;10(6):653-60.
6.Gardner SE, Frantz RA, Troia C, et al. A tool to assess clinical signs and symptoms of localized infection in chronic wounds: development and reliability. Ostomy Wound Manage. 2001;47(1):40-7.
7.Robson MC. Wound infection—a failure of wound healing caused by an imbalance of bacteria. Surg Clin North Am. 1997;77:637-50.
8.Lessing MC, Slack P, Hong KZ, et al. Negative pressure wound therapy with controlled saline instillation (NPWT): dressing properties and granulation response in vivo. Wounds. 2011;23(10):309-19.