
Dr. RJ Ritchie is a certified physician assistant in orthopedic surgery at Lehigh Valley Orthopedic Institute in Allentown, PA. With more than 16 years experience in orthopedic surgery, he works both as a hospitalist in internal medicine and performs per diem urgent care. Dr. Ritchie’s background is in excercise science and athletic training; he received his undergraduate degree and Master of Science in Exercise Science at East Stroudsburg University, PA and his MS in Physician Assistant Studies at DeSales University, PA. His post-graduate research focused on glucosamine chondroitin and effect analysis of obesity in orthopedics. Dr. Ritchie is a paid consultant for 3M.
Ritchie_Current-Dialogues-in-Wound-Management_2023_Article-1
One of the primary issues in post-operative surgical care is wound management, made even more complicated by the myriad medical diagnoses patients concurrently have impeding wound healing. Among the individuals who undergo surgical procedures annually in the United States, 2–4% will develop surgical site infections (SSIs), representing a significant burden on the healthcare system. It has been reported that SSIs account for 20% of all hospital-acquired infections. Furthermore, they are associated with a 2- to 11-fold increase in the risk of mortality, with 75% of SSI-associated deaths directly attributable to the SSI.1 In addition, they are the costliest hospital acquired infection, with an estimated annual cost of $3.3 billion. SSIs extend hospital length of stay by 9.7 days, increasing the cost of hospitalization by more than $20,000 per admission.1
An SSI typically occurs within 30 days post-surgery. The Centers for Disease Control and Prevention (CDC) describes three types of surgical site infections:
- Superficial incisional SSI: This infection is restricted to the area of the skin where the incision was made.
- Deep incisional SSI: This infection occurs beneath the fascia in the muscle and the tissues surrounding the muscles.
- Organ or space SSI: This type of infection can occur in any area of the body other than the skin, muscle and surrounding tissue that was involved in the surgery. This includes body organs or a space between organs.
One of the medical specialties in which superficial and deep incisional SSIs are prevalent is orthopedics. The number of total knee arthroplasties (TKAs) and total hip arthroplasties (THAs) continue to rise annually in the United States. In light of the country’s increasing elderly population, patients are more likely to experience chronic diseases such as diabetes, cardiovascular disease and rheumatoid arthritis, which increase the risk of surgical wound complications. With post-surgical occurrences including soft tissue destruction and improper bony healing (i.e. fractures, hardware infections), wound healing is paramount in achieving positive outcomes in inpatient and outpatient orthopedic surgeries. Surgical site complications (SSCs, i.e. seroma, hematoma, dehiscence, SSIs) following TKAs and TKA revision procedures can potentially increase perioperative mortality rates, costs and the length of hospitalization.1
While the patient is in the clinician’s care, every measure to prevent infection must be taken, including utilizing sterile techniques; antibiotic administration; containing the duration of surgery as much as possible; maintenance of normothermia; proper oxygenation; euglycemia; skin preparation.1 However, infection prevention and promoting healing once the patient is discharged remain major concerns. SSI risk is increased by extensive surgeries, and with total joint arthroplasties being performed increasingly on an outpatient basis, effective measures to ensure proper wound healing are essential.
A highly effective solution to this challenge is the 3M™ Prevena™ Incision Management System, launched in 2010. The system covers and help protects the incision from external contamination, while negative pressure removes fluid and infectious material from the surgical incision. This supports decreased edema via the application of -125 mmHg continuous negative pressure applied by the therapy unit. The device is kept on for up to seven days; though, if allowed, the patient may take a light shower once the therapy unit is turned off and the tubing has been disconnected from the unit, leaving the dressing intact over the incision. It is easily applied in the operating room and is low maintenance to the patient, resulting in a simple solution for incision management in the patient population at increased risk for SSCs. Prevena Incision Management System is the first negative pressure device indicated by the FDA to aid in reducing the incidence of seroma and helping to reduce incidence of superficial surgical site infection in patients at high-risk for post-operative complications in Class I and Class II wounds. However, the effectiveness of Prevena Therapy in reducing the incidence of SSIs and seroma in all surgical procedures and populations has not been demonstrated. Please see full indications for use and limitations at hcbgregulatory.3m.com.
Numerous clinical studies have demonstrated the system’s effectiveness. Clinical studies have reported positive outcomes when using Prevena Incision Management System in patients undergoing TKAs and THAs.2-6 When compared to antimicrobial dressings in the management of revision hip and knee surgery, Prevena Incision Management System use was associated with fewer overall wound complications and fewer total SSIs.2* Similarly, in patients with periprosthetic fracture surgery of the hip and knee, reduced wound complication rates, deep infection rates, and reoperations were observed in the Prevena Incision Management System group compared to patients receiving sterile, antimicrobial hydrofiber dressings.3* A retrospective data review reported reduced rates of SSC in patients at high-risk of developing complications when Prevena Therapy was utilized.5* Doman et al compared use of Prevena Therapy in patients at high-risk of developing SSCs undergoing primary TKA to a historical cohort receiving standard antimicrobial dressings.6 The authors reported a reduction in incisional wound complications and presence of drainage.* Additionally, an increase in non-incision complications (i.e. reaction to dressings, rashes, or blisters) were observed in Prevena Therapy group compared to the control group, though this had minimal clinical impact on patient outcomes and no additional interventions were required. Redfern and colleagues compared prospective patients managed with Prevena Therapy to a historical cohort managed with sterile gauze dressings.4 In the patients receiving Prevena Therapy, reduced rates of SSC, superficial SSI, edema/swelling requiring intervention, and length of stay were observed compared to the historic control group.* Other subjective findings in this analysis found that patients receiving Prevena Therapy reported reduced patient pain 24 hours.4 However, use of Prevena Therapy to help reduce deep SSI, dehiscence, and pain has not been reviewed by the FDA. More study is needed to fully assess the impact of Prevena Therapy use on patient outcomes.
The positive clinical benefits reported by the comparative studies are also mirrored in published randomized controlled trials (RCTs) assessing the impact of Prevena Therapy on SSCs compared to standard of care dressings.7-11 A small RCT reported reduced incidence of seroma and mean seroma volume at postoperative day 10 after THA in the Prevena Therapy group compared to the control group.7 Newman et al reported reduced wound complications, reoperation, and readmission rates in revision knee or hip arthroplasty patients receiving Prevena Therapy.8* An RCT from Cooper et al described a significant reduction in superficial SSIs, and a trend toward lower overall surgical site complications in patients receiving Prevena Therapy compared to use of standard of care dressings among patients at risk of SSCs undergoing anterior total hip arthroplasty.9* Similarly, a multicenter randomized controlled trial (RCT) from Higuera-Rueda and colleagues assessed the use of Prevena Incision Management System in patients after revision knee arthroplasty.10 The incidence of 90-day surgical site complications and hospital readmission rates were significantly decreased in patients receiving Prevena Therapy compared to patients receiving a silver, antimicrobial dressing.10* In addition, Prevena Therapy use reduced the frequency of dressing changes compared with the standard of care.10 A secondary analysis of the Higuera-Rueda RCT reported that patients managed with Prevena Therapy experienced fewer SSCs (3.4% vs 14.3%; P = 0.0013) and required fewer surgical (0.7% vs 4.8%; P = 0.067) and non-surgical (2.7% vs 12.9%; P = 0.0017) interventions compared to those with standard-of-care dressings.11* Despite higher upfront costs for post-operative dressings, Prevena Incision Management System was shown to be cost-effective, decreasing the cost of surgical site management following revision TKA by 49% in the study population and 79% in the higher risk subgroup.11 In light of this combined data, the Prevena Incision Management System continues to be an excellent treatment option in the management of orthopedic incisions during post‑operative care.
In our practice, we have made the clinical decision that all total knee arthroplasty falling under revisions, irrigation and debridement, or any other previous procedures will be managed with the Prevena Incision Management System. Additionally, we have also made intra-operative decisions to initiate the use of Prevena Therapy following incision closure for patients with poor skin quality, severe autoimmune issues that can compromise the skin, and/or morbid obesity. With this approach, we have noticed a decrease in postoperative complications in these scenarios with Prevena Incision Management System use. Representative images of total knee arthroplasty with Prevena Therapy use are shown in Figures 1-3.
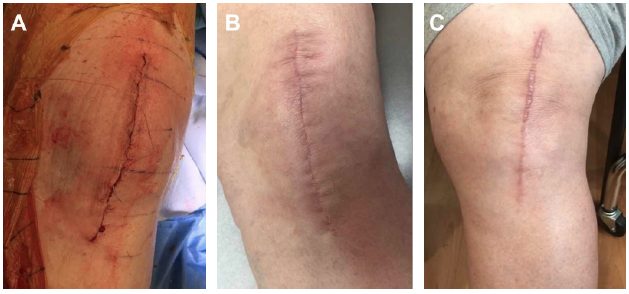
Images courtesy of RJ Ritchie, MS, PA-C, PhD.
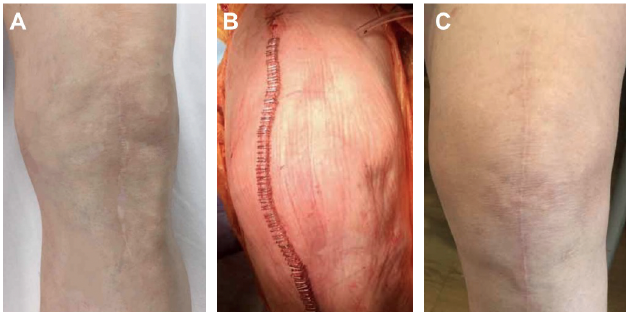
Images courtesy of RJ Ritchie, MS, PA-C, PhD.
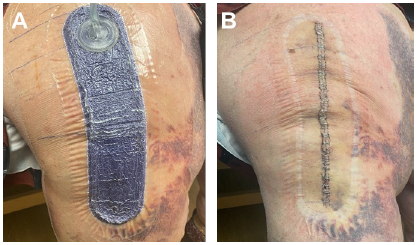
Images courtesy of RJ Ritchie, MS, PA-C, PhD.
* The effectiveness of Prevena Therapy in reducing the incidence of SSIs and seroma in all surgical procedures and populations has not been demonstrated. See full indications for use and limitations at hcbgregulatory.3m.com.
References
- Ban KA, Minei JP, Laronga C, et al. American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 Update. J Am Coll Surg. 2017;224(1):59-74. doi:10.1016/j.jamcollsurg.2016.10.029
- Cooper HJ, Bas MA. Closed-Incision Negative-Pressure Therapy Versus Antimicrobial Dressings After Revision Hip and Knee Surgery: A Comparative Study. J Arthroplasty. 2016;31(5):1047-52.
- Cooper HJ, Roc GC, Bas MA, et al. Closed incision negative pressure therapy decreases complications after periprosthetic fracture surgery around the hip and knee. Injury. 2018;49(2):386-91. doi:10.1016/j.injury.2017.11.010
- Redfern RE, Cameron-Ruetz C, O’Drobinak S, Chen J, Beer KJ. Closed incision negative pressure therapy effects on postoperative infection and surgical site complication after total hip and knee arthroplasty. J Arthroplasty. 2017;32(11):3333-9.
- Anatone AJ, Shah RP, Jennings EL, Geller JA, Cooper HJ. A risk-stratification algorithm to reduce superficial surgical site complications in primary hip and knee arthroplasty. Arthroplast Today. 2018;4(4):493-8.
- Doman DM, Young A, Buller LT, Deckard ER, Meneghini RM. Comparison of Surgical Site Complications With Negative Pressure Wound Therapy vs Silver Impregnated Dressing in High-Risk Total Knee Arthroplasty Patients: A Matched Cohort Study. J Arthroplasty. 2021;36(10):3437-42.
- Pachowsky M, Gusinde J, Klein A, et al. Negative pressure wound therapy to prevent seromas and treat surgical incisions after total hip arthroplasty. Int Orthop. 2012;36(4):719-22.
- Newman JM, Siqueira MB, Klika AK, Molloy RM, Barsoum WK, Higuera CA. Use of Closed Incisional Negative Pressure Wound Therapy After Revision Total Hip and Knee Arthroplasty in Patients at High Risk for Infection: A Prospective, Randomized Clinical Trial. J Arthroplasty. 2019;34(3):554-9. doi:10.1016/j.arth.2018.11.017
- Cooper HJ, Santos WM, Neuwirth AL, et al. Randomized Controlled Trial of Incisional Negative Pressure Following High-Risk Direct Anterior Total Hip Arthroplasty. J Arthroplasty. 2022;37(8S):S931-6. doi:10.1016/j.arth.2022.03.039
- Higuera-Rueda C, Emara AK, Nieves-Malloure Y, et al. The Effectiveness of Closed-Incision Negative-Pressure Therapy Versus Silver-Impregnated Dressings in Mitigating Surgical Site Complications in High-Risk Patients After Revision Knee Arthroplasty: The PROMISES Randomized Controlled Trial. J Arthroplasty. 2021;36(7S):S295-S302.e14. doi:10.1016/j.arth.2021.02.076
- Cooper HJ, Bongards C, Silverman RP. Cost-Effectiveness of Closed Incision Negative Pressure Therapy for Surgical Site Management After Revision Total Knee Arthroplasty: Secondary Analysis of a Randomized Clinical Trial. Journal Arthroplasty. 2022. doi:10.1016/j.arth.2022.03.022

