Jeffrey Litt, DO, is an Assistant Professor of Surgery and Medical Director of the Burn and Wound Program at University Hospital’s Frank L. Mitchell Jr., MD, Trauma Center. He joined MU’s Hugh E. Stephenson Jr., MD, Department of Surgery in July 2014. Born in Iowa but raised in Philadelphia, Dr. Litt completed undergraduate and medical school in Pennsylvania. He and his wife have two children.
Litt_Current Dialogues in Wound Management_2016_Volume 2_Issue 2
Numerous patients with wounds end up receiving skin grafts for wound closure. According to the Agency for Healthcare Research and Quality (AHRQ), in 2012 approximately 240,000 skin grafts were performed in the United States.¹ Many of these are done acutely, as in the case of deep partial- to full-thickness burns or wounds. Chronic wounds may sometimes be grafted to achieve final closure. Finally, many are done reconstructively after removal of cutaneous lesions.
Unfortunately, skin grafting is an operative procedure with concomitant risks. One of those risks is failure of the skin graft to engraft or “take” or achieve reliable vascularization to allow for wound closure. Grafting technique likely plays the largest role in achieving satisfactory “take” through wound bed preparation, harvesting of autograft and graft placement procedures. Graft dressing also plays a significant role; a tightly adherent, conformable dressing helps prevent both shear as well as seroma/hematoma formation, which are both substantial factors in graft loss. Likewise, local wound factors may be contributory, including the presence of significant drainage from the wound, local infection, or non-viable tissue in the wound bed prior to graft placement. Finally, patient factors play a significant role; smokers or other nicotine product users, poorly controlled diabetics, patients on immunosuppressive medications or who are otherwise immunocompromised, patients with post-irradiation treatment wounds, and patients with significant and uncontrolled vascular disease can all experience significant graft failure. Many of these factors can and should be modified pre-operatively, especially in elective cases. What happens, however, when the modifiable elements of the patient and the wound are modified and the skin graft is still threatened?
Basic plastic and reconstructive surgical education tenets conceptualize wound healing as a “reconstructive ladder,” which describes increasingly complex and difficult levels of wound management² or to put it simply, “simpler is better.” Failing skin grafts can also be thought of similarly: the simplest option may be the best and is frequently the best place to start.
The first question when facing the failing skin graft is, “what caused this graft to fail?” A rigorous and objective examination of the site and the patient should ensue, looking for the previously mentioned potential issues. Some causes, such as smoking/nicotine product cessation, halting/lowering immunosuppressant medications, or improving vascular flow, may still improve the graft if intervened upon promptly. Other factors, such as incomplete wound bed preparation or poor initial graft fixation or dressings may doom the graft entirely. Other observations should include the possibility of occult infection or osteomyelitis, the presence of non-viable tissue present or a missed cancer diagnosis.
Infection can be determined clinically on the basis of wound appearance. If any doubt exists, a tissue sample should be sent for a quantitative bacterial count as well as culture and sensitivity. A bacterial count of > 105 CFUs indicates invasive wound infection, likely contributing to graft loss. Appropriate topical antibiosis should ensue. Checking the patient for MRSA colonization should be performed. If positive, adequate methicillin-resistant Staphylococcus Aureus (MRSA) treatment should be started (anecdotally, changing to appropriate topical mupirocin, a monocarboxylic acid antibiotic, halted graft failure in a handful of cases when the cause was otherwise unclear at the initial graft evaluation, but later proved to be related to MRSA involvement). As it may take several days for quantitative cultures to return, adequate skin-coverage with systemic/oral antibiotics would be appropriate until wound culture information has returned–noting that if no clinical improvement is seen and no invasive infection identified they be promptly stopped.
Inpatient or nursing facility patients who have the option of frequent wound care may benefit from “gentle” dressing care involving antibiotic “soaks.” Dr. Carl Moyer at Barnes Hospital in St Louis popularized this method of wound/burn care in the 1960s using a solution of silver nitrate in which he soaked dressings. He periodically moistened the dressings with more of the solution to prevent them from drying. Using these “silver soaks” he reduced burn infection and mortality substantially.³
Unfortunately, “silver soaks” are difficult to manage. By applying the same principle to mafenide acetate, a WWII-era antibiotic, “sulfamylon soaks” came in vogue. Utilizing a 5% mafenide acetate solution, dressings are similarly soaked and used to wrap the region(s) with questionable or fragile-appearing autograft. These dressings are left in place for 48-72 hours and are moistened with additional solution every 4-6 hrs. By leaving these dressings in place, grafts have a chance to engraft in a stronger fashion.
Another ancient topical treatment that has growing modern evidence is honey. Honey has been utilized as a wound dressing for thousands of years in disparate geographic regions – Greece, Rome, Egypt, even the Far East. It is thought of primarily as a bioactive wound dressing and has several important characteristics. First, it is very acidic (pH ~ 3.2 – 4.5), making bacterial growth difficult and increasing oxygen offloading in tissues. It has a high sugar content, which increases wound bed osmolarity, also contributing to its antibacterial qualities. This also improves the wound environment by keeping it moist. Finally, honey has antibacterial qualities due to the presence of hydrogen peroxide and other chemicals. Manuka honey, with which most commercially available wound care products are made, contains the enzyme methylglyoxal instead of hydrogen peroxide, which appears to act synergistically with other unique honey products to form the basis of its antibacterial action.
Matsumura et al in 1998 described a fairly well-known, but rarely seen skin graft complication named “melting graft-wound syndrome”, in which progressive epithelial loss is seen from formerly well-taken autografts and even donor sites. Steptococcus spp were originally thought of as being causative and later Staphylococcus spp were also implicated. Regardless of the cause, honey-based dressings have been shown to be of assistance in regenerating grafts that appear to be affected. The beneficial antibacterial qualities of honey combined with the beneficial anti- inflammatory effects on the wound-bed milieu make for an effective combination. The hydrocolloidal preparation of manuka honey, or MediHoney HCS® (Hydrogel Colloid Sheet) produced by Dermasciences (Dermasciences, Princeton NJ) makes for an easy-to-use and fairly effective dressing regimen for threatened autografts. As it can stay on for several days at a time, it can be considered “gentle,” much like soaks. It also is relatively painless to apply and remove. Anecdotally, the authors have found Medihoney HCS® usage in threatened or frail grafts to be very useful and beneficial.a monoplace chamber or Eustachian tube dysfunction) could benefit from its usage.
The last non-operative approach to assisting with autograft failure would be the usage of hyperbaric oxygen therapy (HBOT). HBOT was initially designed to combat the effects of acute decompression sickness in deep-sea divers. It has been used as a clinical treatment since the early 1900s. As an adjunct to wound healing, it has been used since the 1960s. It works by increasing the oxygen saturation in tissues to assist with oxygen delivery to compromised tissues, such as in wounds. By pressurizing 100% oxygen to 1.5 to 3 atmospheres of pressure, the partial-pressure of dissolved oxygen in tissues can increase to ~ 1500 mmHg with normal being approximately 100 mmHg at sea level in normal, healthy patients. By increasing oxygen delivery to these threatened or infected tissues, it is postulated that macrophage oxygen-dependent killing efficacy is increased. It is also theorized that HBOT stimulates both collagen synthesis as well as angiogenesis and enhances fibroblast function. In addition, the supranormal but localized delivery of oxygen to compromised tissues has been shown in both animal and clinical studies to contribute to both flap as well as graft success. According to the Center for Medicaid and Medicare Services (CMMS), indications for its usage in wounds include compromised grafts or flaps. Therefore, if a hyperbaric chamber were available, any patient with a threatened graft without contraindications (such as uncontrolled seizures, severe uncontrolled asthma, presence of pneumothorax, or relative contraindications such as severe claustrophobia (assuming a monoplace chamber or Eustachian tube dysfunction) could benefit from its usage.
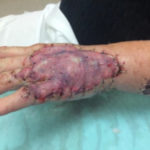
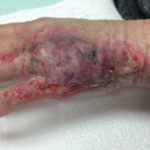
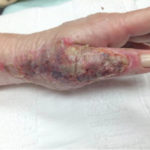
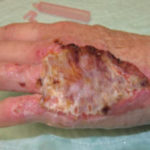
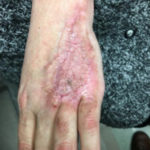
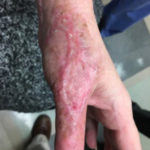
Of course, when all of this fails, a return to the operating room is often appropriate with a keener and more judicious eye of wound bed maximization to allow for better graft take, either immediately or in the future.To summarize, we present a case of a difficult-to-graft hand burn in which numerous modalities were utilized to achieve final engraftment. A 46-year old female was seen after sustaining significant full-thickness burns to her left hand while working in an industrial setting. She was taken to the operating room the following day where her burns were excised and a split-thickness autograft was applied. Standard non- adherent and compressive dressings were applied, as well as a neutral splint. Initial dressing takedown was six days later, where her skin grafts appeared to have near 100% engraftment (Fig 1). She was allowed to perform ROM exercises. Unfortunately, approximately two weeks later her skin graft deteriorated substantially, and her dressing regimen was changed to mupirocin. (Fig 2,3). By the following week her skin graft had failed completely, and underlying tendons were exposed. She was brought back to the OR for re-debridement and placement of a dermal regeneration template (Primatrix Ag, TEI Biosciences, Waltham, MA) (Fig 4,5).
In the OR a tissue sample was sent for quantitative culture and pathology, and her dressing consisted of a silver-based product and a hydrogel to prevent desiccation. Cultures revealed pan-sensitive scant Enterococcus spp, as well as Candida parapsillosis. Her quantitative cultures were unremarkable for invasive infection and a MRSA swab was negative. She was nonetheless started on a two-week course of oral antibiotics as well as a two-week course of high-dose oral antifungal medication. Repeat skin grafting was performed two weeks later. Once again, her grafts looked excellent and then displayed progressive failure at the approximate two and a half to three week mark. Her wound care regimen was changed to MediHoney HCS and HBOT was initiated. Three weeks later her grafts had improved and her wounds were healed. She is now in a scar management program for the hypertrophic scars that have developed (Fig 6,7). Utilizing a multi-modal approach we enabled the wounds to heal. We, unfortunately, still don’t have a clear understanding of the reason(s) for graft failure in the first place. Nonetheless, the patient is healed and is progressing well with OT and non-operative scar modification and ultimately is pleased with her results.
NOTE: As with any case study, the results and outcomes should not be interpreted as a guarantee or warranty of similar results. Individual results may vary depending on the patient’s circumstances and condition.
References
1.Weir LM, Steiner CA, et al (2015). Surgeries in Hospital-Owned Outpatient Facilities. Rockville, MD: Healthcare Cost and Utilization Project, in conjunction with the Agency for Healthcare Research and Quality (AHRQ).
2.Janis JE, Kwon RK, Attinger,CE. The New Reconstructive Ladder: Modifications to the Traditional Model. Plast Reconst. Surg. 2011; 127 (Suppl) 205-12S.
3.Jacobson JS. Burns and Microsurgery. The Greatest Good A History of the John A Hartford Foundation. Danbar Press Inc. 1984. Chapter 13.
4.Molan P, Rhodes T. Honey: A Biologic Dressing. Wounds. 2015;27(6):141-51.
5.Matsumura H, Meyer NA, Mann R, Heimbach DM. Melting Graft-Wound Syndrome. J Burn Care Rehabil. 1998;19:292-5.
6.Boateng J, Catanzano O. Advanced Therapeutic Dresings for Effective Wound Healing – A Review. J Pharm Sci. 2015;104:3653-80.7.Baynosa R, Zambone WA. The effect of Hyperbaric Oxygen on Compromised Flaps. Undersea Hyperb Med. 2012;39(4):857-65.