
Denise Nemeth is a third-year medical student at the University of the Incarnate Word School of Osteopathic Medicine in San Antonio, TX. She completed a Bachelor of Science degree in Biology in 2011 from UT San Antonio, followed by a Master’s Degree in Physician Assistant Studies at UT Health San Antonio. Ms. Nemeth returned to her community to practice as a Physician Assistant (PA) upon graduation in 2015, serving her community as a General and Vascular Surgery PA under the mentorship of three skillful surgeons who trained her to navigate the unique challenges of practicing in the surgical setting in a rural community. During this time, she realized the dire need for well-trained wound care clinicians and embarked on a journey to learn from her peers, receive continuing education, and eventually became the first midlevel provider in her community to be a Certified Wound Specialist with the American Board of Wound Management. Ms. Nemeth currently remains board-certified by the National Commission on Certification of Physician Assistants (NCCPA) and the American Board of Surgical Assistants. She is an active member of the American College of Surgeons, the Latino Surgical Society (LSS), and the Association of Women Surgeons (AWS). She is a member of the Diversity, Leadership and Professional Development (DLPD) and Community Practice Committees of the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). She is currently a Research and Social Media Intern at NYU Langone’s Center for Surgical and Transplant Applied Research (C-STAR). Her research interests are varied and include wound healing, anatomic variations/rarities, and ischemia-reperfusion injuries.

Dr. Jayesh B. Shah is an award-winning wound care and hyperbaric physician in active clinical practice. He is Columbia-trained and certified in Wound Management, Board-Certified in Undersea and Hyperbaric Medicine and Internal Medicine. He is a Fellow of Undersea and Hyperbaric Medicine, and the American College of Physicians and American College of Clinical Wound Specialists. He is a superb educator with extensive speaking experience. He is the President of South Texas Wound Associates, where he has provided clinical wound care services in San Antonio for the past 22 years. He is the President of TIMEO2 Healing Concepts, LLC, which provides consulting and education services in wound care and hyperbaric medicine, and Medical Director of the Wound Healing Center at Northeast Baptist Hospital and Mission Trail Baptist Wound Healing Center in San Antonio, Texas. Dr. Shah is the Past President of the American College of Hyperbaric Medicine, Past President American Association of Physicians of Indian Origin, Past President of Bexar County Medical Society, Current Board of Trustees Member of Texas Medical Association, Current American Medical Association Delegate from Texas, and Current Board Member and Regional Director of International Skin Tear Advisory Panel.Dr. Shah is a paid consultant for 3M Health.
Nemeth_Shah_Current-Dialogues-in-Wound-Management_2024_Article-5
INTRODUCTION
Non-healing surgical wounds are common in hospital and outpatient wound centers. Many acute wounds that arise post‑surgery will heal on their own and will never need to be seen by any wound specialist. However, some surgical wounds will not heal in a timely manner, requiring further evaluation and treatment.
Surgical wound dehiscence is a post-operative complication in which the incision that has been subsequently closed by the surgeon reopens for a variety of reasons. Surgical wound dehiscence can also be referred to as “wound separation”, “wound breakdown”, and “wound disruption”. Regardless of the phrase used, the problem remains the same: an open wound that needs to be closed. This challenge presents a unique set of complications ranging from delayed wound healing to infection, pain, and increased cost of care.
Wound dehiscence can be partial or complete. Partial wound dehiscence occurs when the wound has only dehisced in one or several select areas, and the rest of the incision remains closed and intact. Conversely, in complete wound dehiscence, the entirety of the incision has become dehisced. In the majority of surgical wounds, dehiscence is limited to superficial areas such as the skin and subcutaneous tissue.
Furthermore, in some cases, wounds can dehisce up to muscle and deeper structures and may present with serious tissue compromise, as in the case of evisceration. There is often an offending agent in the wound, such as infection or retained sutures/foreign bodies, that causes an inflammatory process leading to surgical wound dehiscence. In some cases, the dehiscence occurs as the result of direct trauma or strain leading to increased pressure on the area, which can interrupt the healing process. Surgical wound dehiscence can be observed following all types of surgical interventions in any location of the body. The most common non-healing surgical wounds that are seen in wound care clinics are chest wounds following coronary artery bypass graft (CABG) procedures, necrotic breast flaps following plastic surgery, abdominal wounds occurring after herniorrhaphies, caesarian sections, and abdominoplasties. Additionally, some of these surgical wounds have greater complexity as they involve mesh and hardware.Several independent factors have been linked to wound dehiscence including obesity, uncontrolled Type 2 diabetes mellitus (T2D), peri-operative nutritional status, smoking, and chronic steroid use.1 The presence of ascites can also increase the risk of post-operative surgical wound dehiscence at a marked rate.2 Anemia has been reported as a risk factor as it is related to decreased tissue oxygenation and increased peri-operative stress, which can affect the wound healing process.² It is difficult to define the incidence of surgical wound dehiscence, as it would require the inclusion of all surgical procedures performed. However, many clinical studies have done significant work in this field. It is estimated that wound dehiscence occurs in 3.4% of abdominopelvic surgeries; vascular and hernia surgeries have the highest incidence of dehiscence amongst the abdominopelvic surgeries evaluated.1 It is also estimated that surgical wound dehiscence in abdominopelvic surgeries carries a mortality rate of up to 40%.¹
WOUND DEHISCENCE: MANAGEMENT APPROACH
Non-healing surgical wounds are among the most common conditions seen by wound care providers. Myriad factors must be considered when managing the care of a patient with wound dehiscence. Wound care providers should consider that most non-healing surgical wounds typically have an underlying diagnosis preventing the healing process. If a surgical wound remains unhealed for more than 30 days, it is classified as a chronic surgical wound. In such cases, it is important to link the wound etiology to the diagnosis (i.e., T2D, peripheral vascular disease [PVD]; venous, mixed, atypical, etc.). Below, we describe our general approach to the management of surgical wound dehiscence, with the intent that it may help guide other clinicians.
Obtain a complete and thorough patient and surgical history.
When a patient presents for care with a dehisced surgical wound, a detailed patient and surgical history should be obtained (Table 1). Previous imaging and culture tests should be reviewed and repeated if necessary to help determine the appropriate wound care treatment plan based on the patient’s and the wound’s needs.

In certain abdominal wounds, the presence of an enterocutaneous (EC) fistula should be considered. Most EC fistulas can be diagnosed via a thorough patient history and physical examination; however, in some cases, abdomen and pelvis CT scans with fistulogram can aid in diagnosis. Depending on the amount of drainage, EC fistulas can be classified as low output (<500 cc’s of drainage in 24 hours) or high output (>500 cc’s of drainage in 24 hours).
As most wounds require wound bed preparation, we recommend utilizing the TIME principle (Tissue debridement, Infection control, Moisture control; Edge effect, Figure 1).3,4

Adapted image from Sibbald et al.3.
CASE STUDIES
Case Study 1
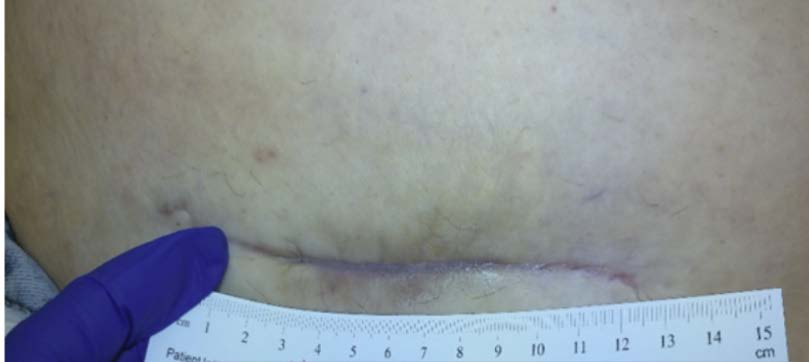
A 32-year-old female was referred to the wound clinic with a dehisced cesarean section incision (Figure 2). The patient had undergone a preterm C-section for fetal head entrapment. Her post-operative course was complicated by endometritis and a multidrug-resistant (MDR) organism. Furthermore, her wound developed a hematoma that contributed to wound dehiscence and was surgically removed prior to referral to our clinic.Previous wound care included negative pressure wound therapy, standard wound care with normal saline irrigation, and wet-to-dry dressings. The patient’s past medical history was relevant for Factor V Leiden deficiency. Antibiotic treatment was initiated by selecting an appropriate agent based on cultures and sensitivity. A computerized tomography (CT) scan of the abdomen and pelvis was performed to rule out infection tracking further into the abdominal cavity. 3M™ V.A.C.® Therapy System using continuous pressure at -125 mmHg was initiated with dressing changes every 72 hours. V.A.C.® Therapy was discontinued after 28 days once the bulk of the wound appeared to be granulated and filled in (Figure 3). The wound care regimen subsequently transitioned to daily application of topical collagen particles, coupled with conservative dressing for three weeks until the wound was completely closed. The dehisced wound was completely healed without complications nine weeks after presentation (Figure 4).
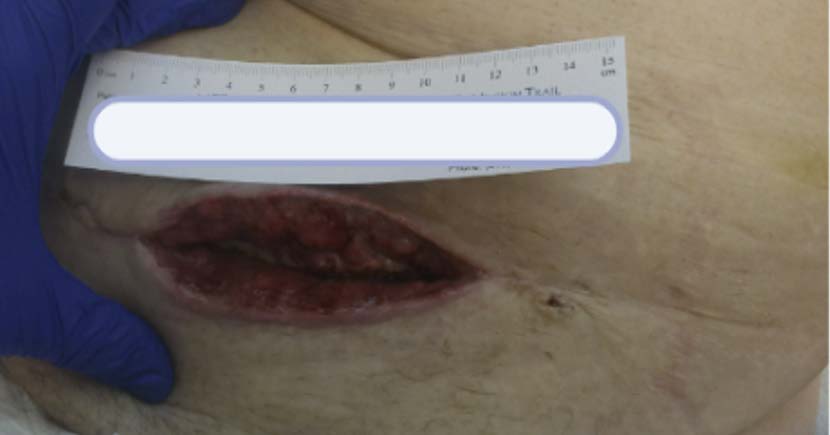
(5.0 cm x 11.0 cm x 6.1 cm).
Image courtesy of Jayesh B. Shah, MD, CWSP and Denise Nemeth, MPAS, CWS.
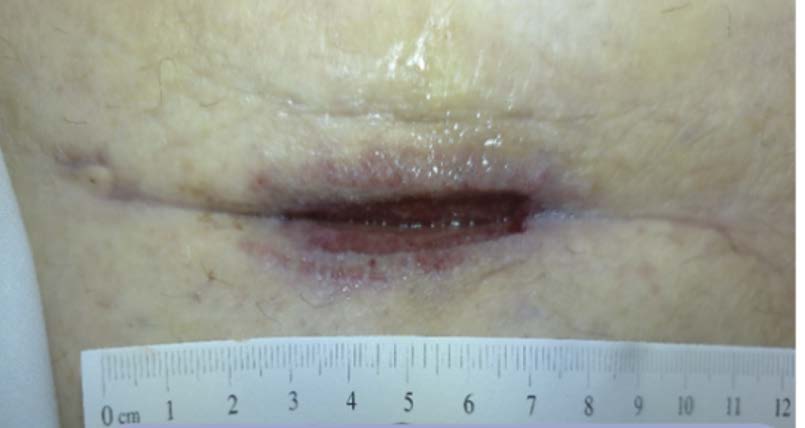
Image courtesy of Jayesh B. Shah, MD, CWSP and Denise Nemeth, MPAS, CWS.
Case Study 2
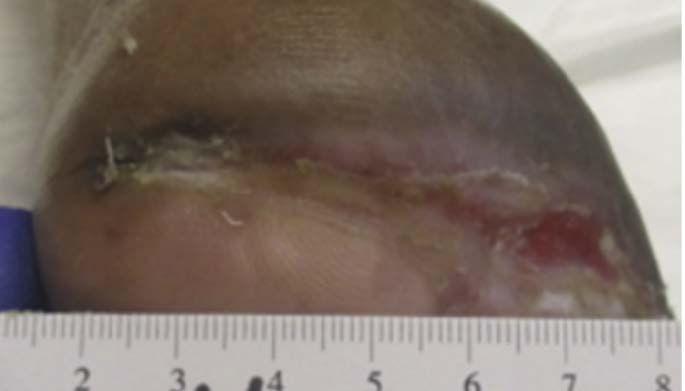
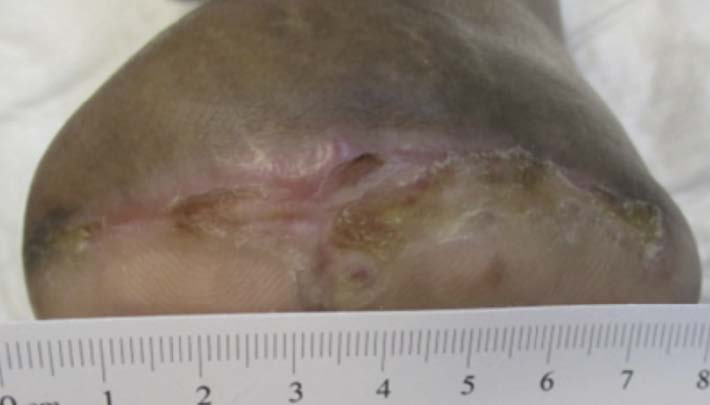
A 67-year-old Hispanic male presented for care with post-operative wound dehiscence following a transmetatarsal amputation (TMA) of the left foot (Figure 5). The patient previously underwent a revascularization procedure to the left lower extremity in an attempt to optimize the blood flow, prevent a TMA, and minimize the possibility of dehiscence in the event of a TMA. Angiography revealed multifocal stenosis of the left posterior tibial, left peroneal, and left anterior tibial arteries. Despite these attempts, the patient’s surgical wound still dehisced. He was sent to our wound care clinic for further evaluation and treatment. Patient medical history included a history of vascular disease, previous osteomyelitis, T2D, and peri-operative anemia. The patient’s hemoglobin A1C was recorded as 7.3%, nearly at goal. Culture and sensitivity showed the presence of pansensitive methicillin-resistant Staphylococcus aureus (MRSA), along with negative blood cultures. Once the presence of infection was confirmed, the patient was referred to the infectious disease physician specialist. Infection-specific antibiotic treatment was initiated. The wound was managed with V.A.C.® Therapy concurrently with hyperbaric oxygen therapy (HBOT). V.A.C.® Therapy System dressing changes occurred every 72 hours. HBOT was utilized daily for a total of 40 consecutive sessions. After seven weeks, V.A.C.® Therapy and HBOT were discontinued for daily wound care with normal saline irrigation followed by application of silver antimicrobial gel and non-adhering dressing with a standard secondary dressing (Figures 6-7). The wound was completely healed 12 weeks after presentation (Figure 8).This patient experienced wound closure; however, he returned to the wound care clinic within seven months for a new non-healing wound on the left foot, which had been treated conservatively for some time prior to referral to our clinic. Further evaluation was provided with a similar approach. The patient’s records were reviewed, and bacterial culture and sensitivity assessments were performed. A Morganella morganii infection was identified, and the patient was referred to an infectious disease specialist for treatment. The patient was also referred to the vascular surgery department for a vascular status evaluation.
Additionally, a surgical consultation was conducted due to exposed bone. A surgical debridement of bone and subcutaneous tissue was performed. This wound was managed using iodinated cadexomer gel and calcium alginate dressings with daily dressing changes. The wound was fully closed within nine weeks of presentation at our wound care clinic.
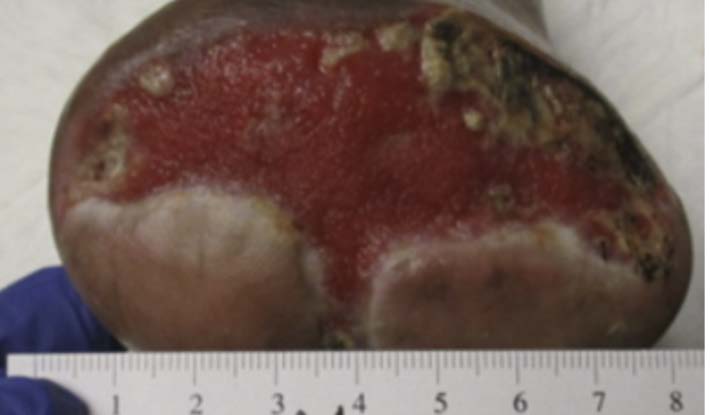
Image courtesy of Jayesh B. Shah, MD, CWSP and Denise Nemeth, MPAS, CWS.
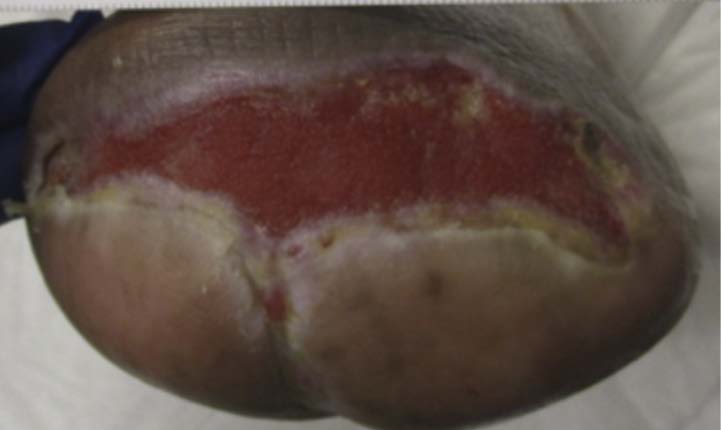
Image courtesy of Jayesh B. Shah, MD, CWSP and Denise Nemeth, MPAS, CWS.
CONCLUSIONS
There is no singular algorithm to treat all patients with wound dehiscence; however, collaboration between surgeons, wound care providers, infectious disease physicians, and additional team members is optimal for obtaining positive patient outcomes. A thorough review of previous records is paramount when caring for patients with surgical dehiscence. An accurate history and physical examination remain the optimal diagnostic tools for understanding the underlying etiologies of non‑healing surgical wounds. The extent of dehiscence determines additional precautions required. Optimization of nutrition, glucose control, management of anemia and vascular status, and infection control are crucial in the care of these patients. Additionally, clinicians should always consider the possibility of an underlying foreign body (i.e., mesh or hardware), as in these situations, the wound will likely remain unhealed unless the foreign body is removed.
References
- Shanmugam VK, Fernandez SJ, Evans KK, et al. Postoperative wound dehiscence: predictors and associations. Wound Repair Regen. 2015;23(2):184-190.
- van Ramshorst GH, Nieuwenhuizen J, Hop WC, et al. Abdominal Wound Dehiscence in Adults: Development and Validation of a Risk Model. World J Surg. 2010;34(1):20-27.
- Sibbald RG, Elliott JA, Persaud-Jaimangal R, et al. Wound Bed Preparation 2021. Adv Skin Wound Care. 2021;34(4):183-195. doi:10.1097/01.ASW.0000733724.87630.d6
- Halim AS, Khoo TL, Saad AZ. Wound bed preparation from a clinical perspective. Indian J Plast Surg. 2012;45(2):193-202. doi:10.4103/0970-0358.101277

