
Stéphanie F. Bernatchez, PhD, is a Senior Research Specialist at 3M. Her work experience includes research and product development in the areas of advanced wound care and skin integrity using in vitro and in vivo assays, as well as clinical research. She received her PhD in Interdisciplinary Sciences from the University of Geneva, Switzerland.

Debra Thayer is a board certified Wound Ostomy and Continence (WOC) Nurse with over 30 years’ experience in the specialty. Prior to joining 3M’s Research and Development team, she provided wound, ostomy and continence care as a clinical specialist in hospitals and post-acute settings. In her current role she works on product and program development and has lectured on skin and wound-related topics across the world. Periwound skin damage, incontinence-associated dermatitis and medical adhesive-related skin injuries related to vascular access are areas of special interest. Most recently she has co-authored 2 chapters for the Wound Ostomy and Continence Nursing Society’s core curriculum textbooks, and an article “Skin Damage associated with Vascular Access: Understanding Common Mechanisms of Injury and Strategies for Prevention” for the Journal of Radiology Nursing.
Thayer_Current Dialogues in Wound Management_2020_Article_21
Introduction
The majority of wound care strategies1-3 are directed toward addressing the wound bed itself. The surrounding skin (periwound) and its role are often ignored in wound healing. The periwound skin provides the proper environment to facilitate healing as the source of epithelial cells needed for wound closure. Dowsett et al4 described the “triangle of wound assessment”: the wound bed, wound edge, and periwound skin are all important factors in the healing of wounds (Figure 1). The purpose of this article is to review periwound skin injury etiology and prevention and treatment methods.
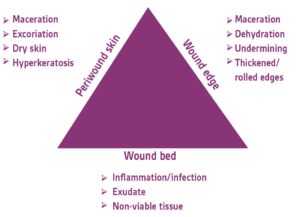
Etiology of periwound skin damage and risk factors
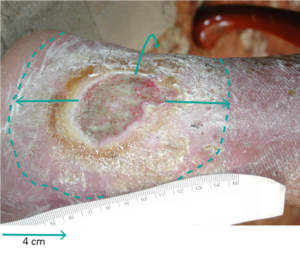
The periwound skin area extends 4 cm beyond the wound edge5 but it should be recognized that damage can extend beyond this zone (Figure 2).4 This area faces a number of challenges during the process of wound care. These challenges are due to the wound itself (intrinsic factors): for example, exposure to exudate from the wound can lead to skin maceration, or wound bed infection can extend to the surrounding tissues. Other contributing factors come from wound care devices (extrinsic factors): wound cleanser products; tapes or dressings used to cover and protect the wound; and devices such as compression bandaging systems, negative pressure wound therapy (NPWT), or offloading devices. Techniques used to apply and remove these various devices are also important to reduce the risk of periwound skin damage.
Intrinsic factors
Frequent challenges in the periwound area include maceration, excoriation, dry (fragile) skin, and hyperkeratosis4 (thickening of the outermost layer of the epidermis). The problems associated with exposure to exudate are termed “periwound moisture-associated dermatitis” and fall under the general category of moisture-associated skin damage.6,7
Skin damage caused by excessive moisture exposure is discussed in several reviews and has been summarized by Voegeli.8 Exposure to moisture impairs epidermal barrier function. Moisture with an alkaline pH reduces the normal function of the skin’s acid mantle.9 Original quantitative studies on human volunteers have shown that skin exposure to water or synthetic urine leads to a significant reduction in skin hardness and a decrease in initial blood perfusion during pressure load when compared to dry sites. In this same study, skin temperature and erythema were lower at wet sites than dry sites.10 Electron microscopy studies showed that extended water contact disrupts the lipid lamellar architecture of the intercellular space and degrades corneodesmosomes (intercellular junctions), which may be the cause of the increased stratum corneum permeability.11
The composition of the exudate differs between acute, healing wounds and chronic, stalled wounds, with elevated levels of proteases and inflammatory cytokines in the latter.12 Bacterial species often found in wounds, such as Pseudomonas aeruginosa, also contribute to protease production in the wound exudate by expressing elastase, which has been shown in vitro to degrade human dermal proteins.13 In a rat model, the authors reported that exposure to moisture containing proteases, followed by bacterial inoculation, induced digestion in the epidermal prickle cell layer. The inoculate caused bacteria-rich clusters to form within the papillary dermis with remarkable tissue damage around the clusters.14 The consensus opinion of an international panel of experts acknowledged these findings and stated that the amount of exudate present and/or its composition may delay or prevent wound healing and cause periwound skin changes.15 This is important because degradation of the periwound skin could not only prevent healing but also lead to wound expansion.
Extrinsic factors
Periwound skin can be damaged by extrinsic factors such as mechanical forces during application, wear, and removal of various devices used in wound care. McNichol et al developed consensus statements for the assessment, prevention, and treatment of medical adhesive-related skin injuries (MARSI).16 Key points included product selection and proper application and removal techniques. A recent (2020) consensus document17 stated that MARSI is overlooked and underestimated, and that anyone is at risk (patients at the extremes of age, patients with dermatological conditions or underlying medical conditions such as diabetes, infection, renal insufficiency, immunosuppression, chronic venous insufficiency). Given these risk factors and the range of products available (from general wound dressings to critical device securement), a judicious product choice is important. A patient may need multiple adhesive device types at the same time. Products that can prevent and/or manage MARSI are described below.
Clinical assessment
The periwound skin clinical assessment typically involves simple visual observations of skin integrity, color, texture, and uniformity of appearance. The epidermis undergoes changes in color and texture upon moisture exposure. Skin becomes white and softens, taking on a saturated or water-logged appearance. While often cited as a challenge to wound healing and management, maceration remains ill-defined.18 Variations in presentation and severity have not been defined and the level that impacts healing is not understood. Documentation of the periwound condition should include measuring the periwound size, noting the skin condition, the exudate presence and characteristics, and the presence of pruritus.19 A periwound skin classification scale has been proposed (HPSC2015), taking into account observations of dessication, maceration, allergic reaction, inflammation, infection, and atypical features. However, the scale requires further validation and has not been widely adopted.20
Quantitative methods have been developed for periwound assessment, measuring skin temperature, amount of edema, epidermal hydration, and erythema. These methods originated in research settings and are not routinely used in clinics yet because they take additional time and equipment and would require a change in practice. Skin temperature measurements have been shown to provide a timely and reliable method to quantify the heat associated with deep and surrounding skin infection and to monitor ongoing wound status. In a study involving 40 participants with chronic wounds (18 non infected and 22 infected), the mean temperature difference between the periwound skin and a contralateral control site was less than 2°F when no infection was present and more than 2°F in the presence of infection.21 A different study22 states that the clinically meaningful temperature difference is 3°F. High-frequency ultrasound has been used to quantify reduction of edema in the periwound tissue in a small group of pressure ulcer (PU) patients on commencement of NPWT. This technology can noninvasively assess the level of edema in wounds and surrounding tissue as well as measure the evolution of granulation tissue in the wound bed without dressing removal.23 Finally, a technique currently used in cosmetics and dermatology to measure epidermal hydration and erythema has been investigated to assess periwound skin and has been found reliable in that context as well (the SD202 skin diagnostic device).24 Future research in clinical settings is needed to determine whether these quantitative techniques should someday be part of the standard assessment methodology for periwound skin.
Prevention of periwound skin damage
Periwound skin damage prevention comprises exudate management and protection of the area surrounding the wound. Uncontrolled exudate can lead to maceration which can be difficult to resolve, especially if skin changes are severe. Avoiding maceration can be accomplished through the use of various products or methods to control exudate production and/or to protect periwound skin.
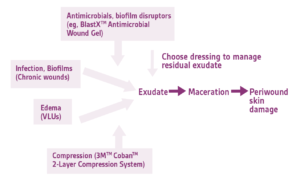
The first step in exudate management is to reduce its production by addressing possible underlying causes, such as infection or edema. Figure 3 illustrates how to first mitigate exudate production before choosing the proper dressing. When large volume exudate is the result of infection, critical colonization, or biofilm, topical and/or systemic antimicrobials can be implemented to reduce microbial load and inflammation (BlastX™ Antimicrobial Wound Gel, a biofilm disruptor). If copious exudate production is due to edema such as in patients with venous ulcers, standard-of-care effective compression (3M™ Coban™ 2-Layer Compression System) can offer the concomitant benefit of exudate reduction.
Once biofilm, bioburden and edema have been addressed, the next step is to ensure proper dressing selection and meticulous application and removal technique. The selection of a dressing with sufficient fluid-handling properties and dressing changes at the appropriate frequency are critical for clinical success. Dressings that are saturated or breaking apart indicate that they are either unable to manage fluid properly or have been left in place too long. The contact surface of the dressing should facilitate rapid vertical wicking of exudate from the wound surface. Exudate should be absorbed and remain in the dressing even under compression. Dressings should be engineered to distribute and evaporate moisture, enabling continuous fluid management during wear. Polyurethane foams are versatile dressings intended to manage minimal to moderate amounts of exudate. They are commonly used as primary dressings for partial thickness wounds or cover dressings over absorbent fillers. Silicone-coated foam dressing (3M™ Tegaderm™ Silicone Foam Border Dressing) can provide a gentle option when an adhesive dressing is desired, or if the periwound or surrounding skin is fragile or painful. For highly exudating wounds, or when dressings must be left in place for extended periods, a superabsorber (KERRAMAX CARE™ Super-Absorbent Dressing) can be an effective cover dressing. Adding an absorbent wound filler (3M™ Tegaderm™ Alginate Dressing; KERRACEL™ Gelling Fiber Dressing) is appropriate for draining wounds with depth. Excessive overlap of wound fillers onto intact skin should be avoided, as this can expose skin to exudate and increase the risk of maceration. Adhesive dressings and tape products should also be applied without tension and removed carefully to avoid MARSI. Manufacturer’s instructions should be consulted for dressing preparation and application technique.
Skin assessment and care are required at every dressing change to maintain periwound skin integrity. If an adhesive product will be used, skin preparation should include clipping of excessive hair to minimize damage at removal. After dressing removal, the wound edge and surrounding skin should be cleansed and assessed. The optimal product for cleansing has not been identified, but saline may be inadequate to rid the skin of dried exudate, residual topicals or dressing debris. Liquid skin cleansers (such as 3M™ Cavilon™ No-Rinse Skin Cleanser) are formulated with surfactants to help loosen soil with minimal friction. To minimize sensitization problems, the ideal cleanser should be pH balanced, fragrance-free, and have a low dermatitis potential (previously referred to as hypoallergenic).
Wound edge protection is an accepted part of wound bed preparation models, yet only a handful of published studies have evaluated interventions.25-27 Polymer-based film-forming barriers provide a beneficial approach for protection of the wound edge and surrounding skin. Alcohol-free liquid films provide breathable and durable skin protection while allowing for visualization of periwound skin. These barriers do not require removal, instead they wear off the skin over time with natural cell turnover and cleansing. Barrier films can also act as a protective interface between the skin and adhesive products. During removal, the film lifts from the epidermis, sparing skin cells and helping to prevent painful stripping injuries. 3M™ Cavilon™ No Sting Barrier Film (Figure 4a) is useful for protection of intact skin from exudate and has been shown to provide a clinical benefit to patients and ease of use for caregivers.25-27 If a barrier film is not available, solid ostomy barrier wafers or hydrocolloids can be applied around a wound using a “picture frame” or “window pane” technique. These products should have a surface coating that allows easy detachment of adhesive drapes, dressings or tape. In contrast to polymer-based film-forming barriers, traditional semi-solid moisture barrier ointments (and some barrier creams) are occlusive and can interfere with moisture vapor transmission from the epidermis, potentially exacerbating maceration. These barriers can contain multi-ingredient formulations increasing the risk of sensitization. This is important for patients with venous ulcers due to their increased risk of allergy to topically applied products.28 Semi-solid barriers removal at dressing change can be uncomfortable for patients, and time consuming for the clinician.

Management and protection of impaired periwound skin
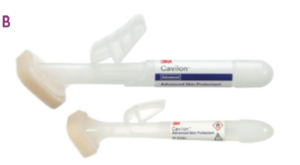
When prevention efforts have failed and periwound skin damage is present, a cyanoacrylate-based elastomeric skin protectant such as 3M™ Cavilon™ Advanced Skin Protectant (Figure 4b) can protect the damaged skin and create an environment for healing. This skin protectant allows adhesive products to adhere on it, therefore the use of other treatment modalities can be continued even when the skin is impaired. The cyanoacrylate component enables attachment to, and protection of moist, damaged surfaces. Early data29 shows promise for use on macerated periwound skin.
Another possible form of periwound skin damage is dermatitis. The presence of dermatitis in periwound skin requires proper evaluation to select the right care. Foroozan et al30 observed that fungal species were present in 27.6% of the skin samples from venous ulcers. If infection is confirmed, the patient may benefit from a topical antimicrobial. On the other hand, dermatitis without infection may be better addressed with a course of corticosteroids.31
When the surrounding skin is intact but dry, moisturizers can be beneficial to prevent the development of dermatitis. Simple, fragrance-free formulations are desirable, and especially important for topical-sensitive populations such as those with venous disease.28
Conclusion
Periwound skin health is important for wound healing and is affected by the way we care for wounds. Various quantitative techniques are now available to assess and monitor periwound skin, but they need further clinical validation before being adopted more broadly. Clinicians should seek products that facilitate wound care while optimizing periwound skin protection. Choosing the appropriate products to manage the exudate, protecting the periwound skin, and ensuring meticulous dressing change technique and frequency are paramount to
promote wound healing.
Acknowledgements
The authors would like to thank Dr Anna Kuang and Ricardo Martinez (3M) for critically reviewing the manuscript and Christina Hernandez (3M) for assistance in figure preparation.
References
1. de Leon J, Bohn GA, DiDomenico L, et al. Wound care centers: critical thinking and treatment strategies for wounds. Wounds. 2016;28(10):S1-S23.
2. Gupta S, Andersen C, Black J, et al. Management of chronic wounds: diagnosis, preparation, treatment, and follow-up. Wounds. 2017;29(9):S19-S36.
3. Jones RE, Foster DS, Longaker MT. Management of Chronic Wounds—2018. JAMA. 2018;320(14):1481-1482.
4. Dowsett C, Gronemann MN, Harding K. Taking wound assessment beyond the edge. Wounds Int. 2015;6(1):19-23.
5. Ferretti DE, Harkins SM. Assessment of periwound skin. In: Milne CT, Corbett LQ, Dubuc DL, eds. Wound, Ostomy, and Continence Nursing Secrets. Philadelphia, PA: Hanley & Belfus, Inc.; 2003:45-48.
6. Gray M, Black JM, Baharestani MM, et al. Moisture-associated skin damage: overview and pathophysiology.
J Wound Ostomy Continence Nurs. 2011;38(3):233-241.
7. Colwell JC, Ratliff CR, Goldberg M, et al. MASD Part 3: peristomal moisture-associated dermatitis and periwound moisture-associated dermatitis. A consensus. J Wound Ostomy Continence Nurs. 2011;38(5):541-553.
8. Voegeli D. Moisture-associated skin damage: An overview for community nurses. Br J Community Nursing. 2013;18(1):6-12.
9. Ayello EA. CMS MDS 3.0 Section M Skin conditions in long term care: Pressure ulcers, skin tears, and moisture-associated skin damage data update. Adv Skin Wound Care. 2017;30:415-429.
10. Mayrovitz HN, Sims N. Biophysical effects of water and synthetic urine on skin. Adv Skin Wound Care. 2001;14(6):302-308.
11. Warner RR, Stone KJ, Boissy YL. Hydration disrupts human stratum corneum ultrastructure. J Invest Dermatol. 2003;120(2):275-284.
12. Trengove NJ, Stacey MC, Macauley S, et al. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen. 1999;7(6):442-452.
13. Schmidtchen A, Holst E, Tapper H, Bjorck L. Elastase-producing Pseudomonas aeruginosa degrade plasma proteins and extracellular products of human skin and fibrolbasts, and inhibit fibroblast growth. Microb Pathog. 2003;34:47-55.
14. Mugita Y, Minematsu T, Huang L, et al. Histopathology of incontinence-associated skin lesions: Inner tissue damage due to invasion of proteolytic enzymes and bacteria in macerated rat skin. PLoS One. 2015;10(9):e0138117.
15. World Union of Wound Healing Societies (WUWHS). Consensus document. Wound exudate: Effective assessment and management. Wounds Int. 2019.
16. McNichol L, Lund C, Rosen T, Gray M. Medical adhesives and patient safety: state of the Science. Consensus statements for the assessment, prevention, and treatment of adhesive-related skin injuries. J Wound Ostomy Continence Nurs. 2013;40(4):365-380.
17. Fumarola S, Allaway R, Callaghan R, et al. Overlooked and underestimated: Medical adhesive-related skin injuries. Best practice consensus document on prevention. J Wound Care. 2020;29(Suppl. 3c):S1-S24.
18. Whitehead F, Giampieri S, Graham T, Grocott P. Identifying, managing and preventing skin maceration: A rapid review of the clinical evidence. J Wound Care. 2017;24(6):159-165.
19. Hunter SM, Langemo D, Thompson P, et al. Observations of periwound skin protection in venous ulcers: A comparison of treatments. Adv Skin Wound Care. 2013;26(2):62-66.
20. Nair HKR. Fifty-patient study evaluating the efficacy of modified collagen with glycerin in periwound skin management. Int J Low Extrem Wounds. 2018;17(1):54-61.
21. Fierheller M, Sibbald RG. A clinical investigation into the relationship between increased periwound skin temperature and local wound infection in patients with chronic leg ulcers. Adv Skin Wound Care. 2010;23(8):369-379.
22. Mufti AS, Somayaji R, Coutts P, Sibbald RG. Infrared skin thermometry: validating and comparing techniques to detect periwound skin infection. Adv Skin Wound Care. 2018;31(1):607-611.
23. Young SR, Hampton S, Martin R. Non-invasive assessment of negative pressure wound therapy using high frequency diagnostic ultrasound: oedema reduction and new tissue accumulation. Int Wound J. 2013;10(4):383-388.
24. Huimin K, Rowledge AM, Borzdynski CJ, et al. Reliability of a skin diagnostic device in assessing hydration and erythema. Adv Skin Wound Care. 2017;30(10):452-459.
25. Coutts P, Queen D, Sibbald G. Peri-wound skin protection. A comparison of a new skin barrier vs. traditional therapies in wound management. Wound Care Canada. 2002;1(1):19,48.
26. Cameron J. Skin care for patients with chronic leg ulcers. J Wound Care. 1998;7(9):459-462.
27. Serra N, Palomar F, Fornes B, et al. Effectiveness of the association of multilayer compression therapy and periwound protection with Cavilon® (no sting barrier film) in the treatment of venous leg ulcers. Gerokomos. 2010;21(3):124-130.
28. Machet L. Sensitization to topical treatments used in leg ulcers: A meta-analysis-(1975-2003) World Union of Wound Healing Societies (WUWHS); 2008; Toronto, Canada.
29. Laforet K, Dias J, Muhammad S. Case series using an advanced silicone-based polymer skin protectant for the clinical management of patients with moisture-associated skin damage (MASD). Canadian Association of Wound Care (CAWC); 2017.
30. Foroozan M, Contet-Audonneau N, Granel-Brocard F, Barbaud A, Schmutz JL. Prevalence analysis of fungi in chronic lower extremity ulcers. Wounds. 2011;23(3):68-75.
31. Rzepecki AK, Blaziak R. Stasis dermatitis: Differentiation from other common causes of lower leg inflammation and management strategies. Curr Geriatr Rep. 2018;7(1):222-227.
NOTE: Specific indications, contraindications, warnings, precautions and safety information exist for these products and therapies. Please consult a clinician and product instructions for use prior to application. Rx only.
© 2020 3M. All rights reserved. 3M and the other marks shown are marks and/or registered marks. Unauthorized use prohibited. PRA-PM-US-02701 (08/20).

