
Kara Couch is a family nurse practitioner and Certified Wound Specialist, who has been working in wound care for the past 12 years. As a nurse scientist, she is an investigator on several clinical trials related to wound healing. Kara also serves as a member of the Guidelines Committee for the Association for the Advancement of Wound Care and has authored or co-authored numerous articles and chapters on wound healing. She also lectures nationally on wound care and wound healing topics.
Couch_Current Dialogues in Wound Management_2015_Volume 1_Issue 2
THERE are many similarities between infection and inflammation in wound care, and they are often mistaken for each other. An example of this is the acute Charcot foot. Typically, patients will present with pain, erythema, edema, and increased warmth to the area. Treatment for cellulitis often starts unless a plain radiograph is also performed and the true culprit is revealed. Antibiotics are not necessary; a plan to calm down the acute inflammation, such as immobilization and limited weight bearing, is indicated.
Venous ulcers account for approximately 70% of all lower extremity ulcers. However, how many of them are truly venous only in etiology? 15-25% of patients also have concomitant arterial disease.1,2 Other conditions account for approximately 15% of the etiology.1Inflammatory conditions, including vasculitis and vasculopathy, can mimic a venous ulcer, but their treatment plans are often quite different. Taking a careful medical history and a review of symptoms can help to differentiate between conditions. Patients whose ulcers fail to improve after 4 weeks of standard of care for a venous ulcer need re-evaluation for other etiologies.3A skin biopsy can be very useful in elucidating the underlying problem. An autoimmune and a prothrombotic laboratory panel are also indicated for diagnostic assistance.
Conditions associated with an inflammatory wound include rheumatoid arthritis, systemic lupus erythematosus, atrophie blanche, inflammatory bowel disease (i.e., Crohn’s disease or ulcerative colitis), hepatitis C, and pyoderma gangrenosum (PG). The hallmark of treatment for these conditions is to treat the underlying disease systemically and then topically address the wound bed appropriately. Once the underlying disease is quiescent and stable, more aggressive treatment options, including debridement, are possible. Failure to treat the underlying disease medically can result in pathergy. This is known for disease processes such as PG. Recent advances in immune-modulating therapies have been very successful in treating these ulcers.
An infected leg ulcer typically has some classic signs of infection, including increased pain, malodorous exudate, periwound erythema and edema, and nonviable tissue in the wound bed.4 Inflammatory wounds can also have some of these characteristics, but there are distinct differences.
PAIN
Pain is a way to help differentiate between an inflammatory and an infected wound.5Inflammatory wounds are often shallow, but the associated pain is distinctly out of proportion to the ulcer size. For example, a small ulcer of less than 1 cm2 can be intensely painful. Patients will describe a stabbing or twisting pain as if a hot poker is being put against their skin. Although some venous ulcers are very painful, the pain is more diffuse. Additionally, pain from an infection can affect an entire extremity, whereas it is more localized with an inflammatory wound.
ERYTHEMA
Erythema related to inflammatory wounds is localized to the wounds themselves.5 However, erythema from infection tends to be expansive and can spread both distal and proximal to the affected site. Both can exhibit warmth.
LESIONS
Ulcers that are consistent with inflammatory conditions often have multiple lesions closely located around the primary ulcer.5 In infected ulcers, there is often only a single ulceration that is present.
Case 1 is a 27-year-old male with numerous extremity ulcers for 10 months. He also has inflammatory arthritis with fused wrists and hepatitis B as comorbidities. It was a complex case, as he needed antiretroviral therapy for the hepatitis B prior to initiating immunosuppressing medications. He is being treated with monthly injections of ustekinumab as well as tenofovir to keep his hepatitis suppressed. His topical therapy is relatively simple due to insurance challenges. We are using non-contact low frequency ultrasound as well as Xeroform several times a week. The patient also uses hypochlorous acid as an antiseptic agent with dressing changes outside of clinic. He has improved greatly with the ustekinumab and will be placed on a maintenance regimen once all ulcerations are closed (Figures 1A and 1B).
Case 2 is a 26-year-old male with bilateral medial malleolar ulcers. He also had a history of chronic deep vein thrombosis to bilateral lower extremities as well as bilateral equinus deformities from the ulcers. The ulcers are intensely painful and make dressing changes/wound care challenging. A thorough autoimmune and prothrombotic panel was ordered. The patient was diagnosed with mixed connective tissue disease, and the ulcers are vasculitic in origin. He is being treated with anticoagulation therapy, as well as an immune-modulating agent to tamp down his system. Topical therapy is focused on decreasing fibrin buildup as well as superinfection (Figures 2A and 2B).
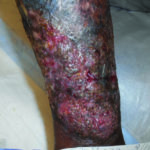 Figure 1A. Pyoderma gangrenosum to the lower leg of a 27 year old
Figure 1A. Pyoderma gangrenosum to the lower leg of a 27 year old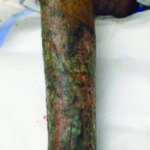 Figure 1B. After treatment with ustekinumab monthly
Figure 1B. After treatment with ustekinumab monthly
Case 3 is a 70-year-old female with a left distal medial calf ulcer from atrophie blanche associated with livedoid vasculopathy (LV). Livedoid vasculopathy is a thrombo-occlusive disease.6 She has had ulcerations that have waxed and waned over the years, and a new ulcer developed 3 months ago. She is being treated with non-contact low frequency ultrasound twice weekly as well as daily hydrogel. In addition, she is using enoxaparin and pentoxyfylline7, which are treating the LV. Atrophie blanche ulcers have classic atrophic white satellite scarring with hyperpigmentation. This patient also has chronic venous disease and will be undergoing stab phlebectomies to assist with wound healing. She is intolerant of compression due to pain (Figure 3).
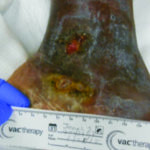 Figure 2A. Left medial ankle ulcers due to vasculitis and mixed connective tissue disease
Figure 2A. Left medial ankle ulcers due to vasculitis and mixed connective tissue disease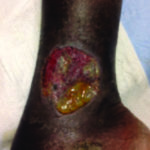 Figure 2B. Right medial ankle ulcers due to vasculitis and mixed connective tissue disease
Figure 2B. Right medial ankle ulcers due to vasculitis and mixed connective tissue disease
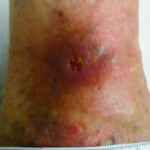 Figure 3. Ulcer due to atrophie blanche
Figure 3. Ulcer due to atrophie blanche
Case 4 is a 53-year-old man with a past history of hidradenitis suppurativa and emphysema who developed a left proximal tibial ulcer over a 3-week period. It initially started as a pustule and then rapidly expanded. He was admitted to the hospital with cellulitis but failed to respond to antibiotic therapy. He underwent punch biopsy by dermatology. He was diagnosed with PG and treatment commenced with topical and systemic steroid therapy (Figure 4).
In summation, a careful history and diagnostic evaluation can determine if an ulcer is inflammatory in etiology. Inflammatory ulcers require treatment of the underlying disease process in addition to meticulous local care. Infected ulcers should improve dramatically over a short period of time with effective antibiotics (topical and systemic) and aggressive debridement. Inflammatory ulcers require both longer-term systemic therapy with immune-modulating agents as well as local wound care targeted to improve the wound bed and overall healing. Debridement may be delayed until disease stabilization or of an autolytic variety, which is gentler to the tissue so as not to promote an inflammatory wound bed.
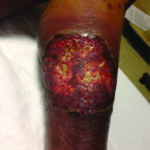 Figure 4. Pyoderma gangrenous that has a super infection with staphylococcus aureus
Figure 4. Pyoderma gangrenous that has a super infection with staphylococcus aureus
References
1.O’Donnell TF, Passman MA, Marston WA, et al. Management of venous leg ulcers: Clinical practice guidelines of the Society for Vascular Surgery® and the American Venous Forum. J Vasc Surg. 2014;60(2):3S-59S.
2.Marston W. Mixed arterial and venous ulcers. Wounds. 2011;23(12):351–6.
3.Brem H, Kirsner RS, Falanga V. Protocol for the successful treatment of venous ulcers. Am J Surg. 2004;188(1),S1:1–8.
4.Sibbald RG, Goodman L, Woo KY, et al. Special considerations in wound bed preparation 2011: An update. Adv Skin Wound Care. 2011;24(9):415-36.
5.Woo KY, Sibbald RG. Chronic wound pain: A conceptual model. Adv Skin Wound Care. 2008;21:175–88.
6.Alavi A, Hafner J, Dutz JP, et al. Atrophie blanche: Is it associated with venous disease or livedoid vasculopathy? Adv Skin Wound Care. 2014;27(11):518-24.
7.Parsa H, Zangivand AA, Hajimaghsoudi L. The effect of pentoxifylline on chronic venous ulcers. Wounds. 2012;24(7):190-4.

