
Dr. Lucian G. Vlad currently serves as an Assistant Professor at Wake Forest School of Medicine in the Wound Care and Hyperbaric Medicine Center in Winston-Salem, North Carolina. Dr. Vlad graduated Medical School in 2001 from the University Lucian Blaga in Sibiu, Romania. He completed his residency in 2008 at the Grand Rapids Medical Education & Research Center. He is board certified in family medicine and from 2008 until 2012 Dr. Vlad worked in an outpatient family medicine practice and also worked as a hospitalist. Since 2012, Dr. Vlad has been a faculty member in Plastic Reconstructive Surgery at Wake Forest School of Medicine and has been a full-time clinician in the outpatient Wound Care and Hyperbaric Medicine Center. His research interests include collagen matrices, low intensity laser therapy, cellular therapy, and heat therapy. Since 2017, Dr Vlad has been on faculty for the wound care fellowship offered at Wake Forest University. Dr. Vlad is a consultant for 3M.
Vlad_Current-Dialogues-in-Wound-Management_2021_Article_6
Venous leg ulcers (VLUs) are one of the most common issues encountered in a wound care clinic.1 The prevalence of VLUs is estimated to be 2% of the population worldwide and this increases to 5% in people over the age of 65 years old.2,3 VLUs are considered a major healthcare expenditure involving many resources, multiple specialties, multiple treatment options, compression bandages, and multiple doctor visits.4 VLUs contribute to lost productivity with a 29% increase in work-loss costs compared to non-VLU patients.1 Complications in VLUs includes cellulitis and other soft tissues infections that can often be of mild to moderate severity. Higher severity infections can lead to sepsis, hospitalizations, multiple surgeries and even death. VLUs are also associated with a high psychological impact of isolation, embarrassment, negative emotions anxiety and depression.1,3
The pathophysiology of VLUs is complex and is primarily driven by an increase in venous pressure. Perfusion at the level of tissue is driven by the difference between the arterial and venous side of the capillary bed. Increased venous pressure results in areas of “stasis” where drainage of the venous and lymphatic system is diminished. These areas are targets for VLU development. The most common stasis area is located on the leg around the malleolar area and distal half of the leg also known as “gaiter area”.
Venous hypertension is a wide spectrum disorder. It can cause symptoms in any individual at any age. A healthy individual without any risk factors for venous disease will develop physiologic venous hypertension by simply standing. But in context of intact calf muscles and competent veins, the increased venous hypertension is quickly relieved within a few steps (contractions of the calf muscles) or by leg elevation. Most people have experienced a sense of relief by elevating their legs after a day of work. These transient episodes of elevated venous pressure do not produce significant physiologic or pathologic effects.
However, over long periods of time and multiple and repeated episodes of elevated venous hypertension, these effects start to slowly appear. Initially telangiectasia, reticular veins, and varicose veins can appear. The issues caused by these are largely cosmetic in nature.
More advanced stages of VLU development involve skin pigmentation, then stasis dermatitis. These clinical findings are driven by tissue changes consisting of endothelial dysfunction, leakage of leukocytes and red blood cells in the interstitial space, increased tissue inflammation, activation of proteolytic activity, hemosiderin deposits, and metabolic alterations. As the disease progresses, additional symptoms like burning, stinging, skin flakiness, desquamation, cramps, and pain may manifest themselves. In advanced stages, lipodermatosclerosis, fibrosis panniculitis, atrophie blanche, or livedoid vasculopathy may also be present in patients with VLUs. The duration of these changes is both slow and long; usually a minimum of 20-30 years which is why the incidence of VLU increases with age.
Ultimately, the end stage of venous hypertension is the venous leg ulcer. The healing of a VLU is a slow process, and many become refractory to treatment. When healing occurs, there is a high recurrence rate. With adequate care, healing rates of 76% have been documented at 16 weeks in the published literature and recurrence rates are estimated at 50-70% at 6 months.4,5
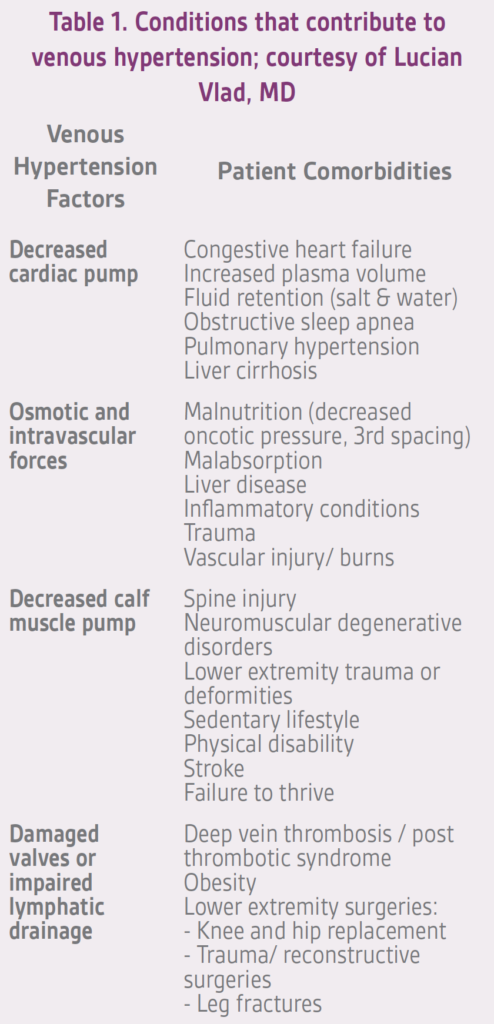
Treatment of a venous leg ulcer is quite complex. It is critical to address the underlying factors that contribute to venous hypertension (Table 1), but many other issues also need to be considered such as adequate debridement, dressing selection, infection management, differential diagnosis, etc. However, in this article the focus will center on practical issues related to compression therapy for VLU management.
Compression therapy is one of the only practical options to counteract the harmful effects of gravity that causes increased venous pressure, dilation and deformation of vein, valvular incompetence, stretching and decreased elasticity of the skin, fascia, lymphatics, and sponge like transformation of dermis and subcutaneous tissue.6 Compression therapy is considered the gold standard and the most important therapy for patients with VLU. As per the 2014 Society for Vascular Surgery and the American Venous Forum Guidelines, compression therapy is the only item with a Grade 1A recommendation and level of evidence.7 It has been demonstrated to effectively reduce several inflammatory and proteolytic biomarkers.8,9
Clinicians should take note that compression therapy is difficult to tolerate, and patient compliance is a challenge. While the majority of patient compliance issues can be avoided with adequate application of the therapy, patient education and adequate supervision of the therapy, it may be worthwhile reviewing some important techniques to enhance patient acceptance.
Prior to starting compression therapy, a perfusion assessment must be done.7 Arterial ankle pressure and ankle brachial index (ABI) must be considered before recommending a compression gradient for a patient.
There are many types and brands of compression garments available for patients such as compression stockings, single layer and multilayer wraps, elastic (long stretch) and inelastic (short stretch). Unfortunately, there is no consensus or evidence as to what type of compression delivers the best outcomes for VLU management. Patient compliance with compression stockings is abysmally low. Many patients have difficulty putting on and taking off stockings due to their inability to reach their feet (e.g., obesity, hip or knee surgeries, back pain, etc.), or due to decreased grip strength. In my opinion, compression stockings should be considered as prevention to leg swelling and VLU and not for treatment. The presence of VLU requires stringent continuous compression which usually be achieved only with multilayer wraps that are changed in a clinic by trained wound care professionals.
The literature on compression use for VLUs is clear on a number of issues:5,10
- Multilayer systems are more effective than single layers
- Higher pressures are more effective than lower pressure
- Inelastic compression is superior to elastic systems
Some of the recent (5-10 years) research findings have characterized the difference between resting pressures (inactivity) and working pressures (activity; e.g. walking). The goal of a compression wrap is to deliver low resting pressure and high working pressures. It is logical that multilayer wraps work better, as more layers mean increased stiffness.
Some of the things to consider increasing the success of compression therapy in your VLU patients:
- Patient education is critical. VLU and venous hypertension can be hard to grasp not just for the patient by also for healthcare professionals. Important elements to convey to the patient include the concept of “stasis” and engaging the patient in the attempt to improve the situation. Patients should be counselled to focus on ankle flexion extension exercises at a minimum and encouraged to increase physical activity and walking. Leg elevation, when possible, is also encouraged, thereby allowing gravity to work in the patient’s benefit rather than against the patient. To this end, recommending the incorporation of 10-15 minutes of leg elevation every hour during the daytime can make a significant difference.
- Paying close attention to concomitant medications that can cause fluid retention or leg swelling such as calcium channel blockers, thiazolidinediones, nonsteroidal anti-inflammatory drugs, corticosteroids, and insulin is important.
- Intense inching and burning sensation caused by stasis dermatitis is one of the most common symptoms that impact patient adherence to compression therapy. If the dermatitis is moderate or severe, a course of topical low potency steroids to decrease these symptoms before multilayers wraps can be tolerated may be required.
- Appropriate dressing selection can greatly impact the success of compression therapy Dressings must be chosen to capture wound drainage for the duration of the utilization of compression wraps and to protect the periwound skin. Liberal use of barrier skin on dry periwound skin is strongly encouraged. High exudative wounds may require wrap changes every 2-3 weeks for 1-3 weeks. With stringent compression, the expectation is that drainage will decrease.
- Gradual compression concept: Many patients do not tolerate adequate compression in the therapeutic range upon presentation in the clinic due to wound pain, drainage amount, skin irritation, and stasis dermatitis among many factors. In these situations, it may be useful to start with a self-removable low compression garment (e.g. 8-12 mmHg for 1-2 weeks). This will allow treatment of stasis dermatitis if needed. After 1-2 weeks, the patient can be transitioned to a lower pressure garment (e.g. 20-25 mmHg system for 1-2 weeks). The frequency of changes can be adjusted as needed from once per week to 3 times per week. After 2 more weeks, the patient can be transitioned to a conventional compression garment with pressure in the 40-50 mmHg range. Through personal experience, this clinical management approach has resulted in many “refractory” VLU patients becoming compliant patients that can heal.
- Staff training is, perhaps, the most important aspect of compression therapy. Consistent application and troubleshooting by experienced staff can greatly enhance adherence to treatment.
In conclusion, compression therapy is a significant addition to the clinician’s armamentarium in treating the epidemic of VLU. Patient education, provider understanding of the disease process, and staff training are key elements for the successful implementation of a compression therapy treatment algorithm.
References
1. Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons N. Burden of venous leg ulcers in the United States. J Med Econ. 2014;17(5):347-356.
2. Chi YW, Raffetto JD. Venous leg ulceration pathophysiology and evidence based treatment. Vasc Med. 2015;20(2):168-181.
3. Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circulation. 2014;130(4):333-346.
4. Raffetto JD, Ligi D, Maniscalco R, Khalil RA, Mannello F. Why Venous Leg Ulcers Have Difficulty Healing: Overview on Pathophysiology, Clinical Consequences, and Treatment. J Clin Med. 2020;10(1).
5. O’Meara S, Cullum N, Nelson EA, Dumville JC. Compression for venous leg ulcers. Cochrane Database Syst Rev. 2012;11(3):CD000265.
6. Partsch H, Mortimer P. Compression for leg wounds. Br J Dermatol. 2015;173(2):359-369.
7. O’Donnell TF, Jr., Passman MA, Marston WA, et al. Management of venous leg ulcers: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2014;60(2 Suppl):3S-59S.
8. Beidler SK, Douillet CD, Berndt DF, Keagy BA, Rich PB, Marston WA. Multiplex analysis of matrix metalloproteinases in leg ulcer tissue of pateints with chronic venous insufficiency before and after compression therapy. Wound Repair Regen. 2008;16(5):642-648.
9. Beidler SK, Douillet CD, Berndt DF, Keagy BA, Rich PB, Marston WA. Inflammatory cytokine levels in chronic venous insufficiency ulcer tissue before and after compression therapy. J Vasc Surg. 2009;49(4):1013-1020.
10. Stücker M, Link K, Reich-Schupke S, Altmeyer P, Doerler M. Compression and venous ulcers. Phlebology. 2013;28(Suppl 1):68-72.
© 2021 3M. All rights reserved. 3M and the other marks shown are marks and/or registered marks. Unauthorized use prohibited. PRP-PM-US-03007 (03/21).

