Wound Healing Unit, Montpellier University Hospital
NOTE: As with any case study, the results and outcomes should not be interpreted as a guarantee or warranty of similar results. Individual results may vary depending on the patient’s circumstances and condition.
INTRODUCTION
Removing sloughy tissue in wounds can be challenging for many clinicians. Clinical assessment of wound depth and undermining is a skill that is poorly acquired, owing to a lack of understanding of the pathophysiological determination of wound infection. Surgical wound debridement can be tedious and sometimes considered a “second class” surgery that clinicians delay or avoid. Additionally, in certain patients, complete surgical debridement may not be appropriate and may even be contraindicated based on various patient and wound factors.1 For a variety of reasons, some patients present with critical complications due to the persistence of high bacterial burden.
Negative pressure wound therapy with instillation of fluids and a dwell time (NPWTi-d) is an advanced wound management modality that has been found to help manage bioburden in complex wounds2,3 or when there is failure of conventional NPWT.4-7 The therapy facilitates automatic instillation of topical solutions that are cyclically fed into the foam dressing via an additional set of tubing and held for a user-selected period before removal by negative pressure. The combination of topical wound solutions and NPWT assists with wound cleansing and removal of wound exudate and infectious material.
A reticulated open-cell foam dressing that includes an array of through holes in its wound contact layer (ROCF-CC; V.A.C. VERAFLO CLEANSE CHOICETM Dressing, KCI, an ACELITY Company, San Antonio, TX) is the latest iteration of NPWTi-d.8 The foam dressing is used adjunctively with NPWTi-d and consists of two foam layers: a wound contact layer with 1.0 cm diameter holes spaced 0.5 cm apart and a cover layer without holes. Adjunctive use of NPWTi-d with ROCF-CC provides for immediate cleansing of wounds that may contain slough and/or and non-viable tissue when surgical debridement is not immediately available or may not be clinically appropriate.8 The dressing is meant to help facilitate the removal of thick wound exudates such as fibrinous materials and slough when used with NPWTi-d. We have been using this new technology at Montpellier University Hospital (Montpellier, France) since 2015 to assist in removal of nonviable tissue when we are not able to take patients into the operating room.
BACKGROUND
The first commercially available device combining NPWT and intermittent instillation of solutions was introduced in 2002.9 Wolvos (2004) first reported on the outcomes using this device with a variety of solutions, using a dwell time of 5 minutes and the resumption of negative pressure for 3 hours at −125 mmHg.9Automated instillation with NPWT was developed to create a controlled, protected environment for flushing and cleansing wounds by the proposed mechanism of loosening soluble contaminants in the wound bed followed by subsequent removal during NPWT.10,11 A next-generation NPWTi-d device was introduced in 2011 that allows the clinician to visually determine the correct instillation volume, perform a test cycle to confirm appropriate settings and soak the dressing with a topical solution prior to dressing removal.5 In 2013, a panel of experts published international consensus guidelines designed to address the appropriate use of NPWT with intermittent instillation.12 More recently, an updated review of the evidence and recommendations for use of NPWTi-d were published by an expert working group.13 In these recommendations, normal saline was recommended as the preferred solution to instill with NPWTi-d, based on evidence demonstrating that normal saline can achieve outcomes similar to other types of solutions.2,4,5,7,13 The device settings we have used in our institution for NPWTi-d with ROCF-CC are within the parameters set forth in the 2015 review, which suggested a cycle frequency of 2 – 4 hours at −125 mmHg and a dwell time of 10 – 20 minutes.
PRACTICAL SUGGESTIONS FOR USE
Based on our hospital’s experience with the new ROCF-CC dressing, we have developed some practical advice for clinicians to maximise use of NPWTi-d with ROCF-CC to help achieve optimal patient outcomes:
1)Users should maintain good understanding of the foam characteristics: Three pieces of foam are included in each dressing kit: one contact layer with through holes (18 x 12.5 x 0.8cm) and two cover layer foams of two different thicknesses: 0.8 and 1.6 cm. The through holes in the ROCF-CC contact layer measure 1.0 cm in diameter and are spaced 0.5 cm apart. When wet, ROCF-CC foam has a tensile/tear strength that is three times greater than a wet standard reticulated open-cell foam dressing (ROCF-V; V.A.C. VERAFLO™ Dressing, KCI, an Acelity company, San Antonio, TX). ROCF-CC is more absorptive (less hydrophobic) than ROCF-V, and exudate viscosity removal is up to 30 centipoise.
2) Large pieces of hard necrotic tissue should be debrided prior to use of ROCF-CC.
3) NPWTi-d with ROCF-CC should not be usedprior to initiation of antibiotics when bone infection is confirmed with a positive probe. A bone biopsy should be performed after debridement to confirm the presence of bacteria and their sensitivities to antibiotics as well as the presence of bone tissue necrosis. In cases of a positive bone biopsy, an MRI may be prescribed to confirm the presence of osteitic bone. When an infection is confirmed, culture-specific antibiotics should be initiated.
4) The ROCF-CC dressing can be applied to all wounds in a similar manner
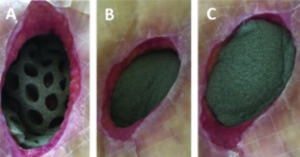
A. Placing the dressing: The wound contact layer with through holes is cut to size and placed completely into the open wound, over the surface of the wound bed (Figure 1A). The cover layer (without holes) is placed over the wound contact layer to cover the wound contact layer as well as to fill the undermined areas around the wound. A second cover layer provided in the dressing kit can be used for filling deep wounds (Figure 1B,C).
B. Placing the drape and starting instillation:An adhesive drape is placed over the two (or three) foam layers, and instillation tubing is applied to the dressing as appropriate and connected to the NPWTi-d device.
C. Initiating NPWTi-d: Our facility has experienced good outcomes with saline instillation every 3.5 hours with a dwell time of 10 minutes. If the desired results are not obtained with saline, other topical solutions can be considered and applied in accordance with manufacturer’s recommendations.9The recommended level of negative pressure is −125 mmHg, and dressings should be changed every 2-3 days.
D. Dressing changes: At each dressing change, the contact layer and the cover layer foams are removed along with sloughy tissue, the wound is manually washed with saline, and a new dressing is applied.
E. Pain relief management (10 mg oral morphine) may be administered prior to dressing removal if needed. The pain associated with the dressing change pain usually increases following the rapid progression of granulation. Usually when necrotic tissue and fibrin are still present, some pain is observed.
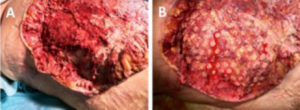 Figure 5: Large post-surgical wound (after necrectomy of infected calciphylaxis) at presentation (A). One section of the wound was covered with ROCF-CC and the rest of the wound was covered with a standard ROCF-V foam dressing; columns from the ROCF-CC were visible on the wound at first dressing change(B).
Figure 5: Large post-surgical wound (after necrectomy of infected calciphylaxis) at presentation (A). One section of the wound was covered with ROCF-CC and the rest of the wound was covered with a standard ROCF-V foam dressing; columns from the ROCF-CC were visible on the wound at first dressing change(B).
F. Extreme care should be taken not to leave any piece of ROCF-CC dressing in the wound after dressing removal, particularly because of the way in which granulation tissue develops through the holes in the wound contact layer. Figure 2A shows penetration of granulation columns through the holes of the wound contact layer after three days of being in place, prior to dressing change, and Figure 2B shows the typical depth of the columns immediately after foam removal.
CLINICAL EXPERIENCE AND CASES
In our experience at our institution, approximately 95% of wounds have displayed rapid granulation tissue formation underneath the wound contact layer foam. This ‘sprouting’ has been observed at the first dressing change at 3 days, and through to 9 days (3 dressing changes). We have noted that most of the non-viable tissue is removed at the first dressing change after 3 days of therapy. In most of the cases, the wound bed contains ≤10% devitalised tissue at the third dressing change after 9 days of therapy, with a rapid decrease of the necrotic/fibrinous tissue. Figures 3-5 display typical progression of wounds underneath the ROCF-CC dressing.
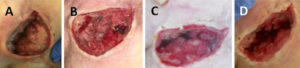 Figure 4: Evolution of a sacral pressure ulcer treated with NPWTi-d and ROCF-CC from presentation (A). First dressing change at 3 days (B). At 6 days (C). At 9 days, undermining is reduced and wound is granulated (D).
Figure 4: Evolution of a sacral pressure ulcer treated with NPWTi-d and ROCF-CC from presentation (A). First dressing change at 3 days (B). At 6 days (C). At 9 days, undermining is reduced and wound is granulated (D).
Figure 6 shows removal of the ROCF-CC wound contact layer along with layers of nonviable tissue from a sacral pressure ulcer with various tissue depths. During use of NPWTi-d with ROCF-CC, holes in the contact layer foam were rapidly filled with red ‘islands’ of granulation tissue covered with white/yellow fibrin and non viable tissue (Figure 4A). The base of the wound bed apart from the islands, or columns, was more rapidly cleansed of the undesired tissue (Figure 4B). A soak feature of the NPWTi-d device allowed easier removal of the contact layer (Figure 4C). Slough and other nonviable tissue were removed with the contact layer (Figure 4D).
CLINICAL INDICATIONS
In our experience, the clinical indications for NPWTi-d with ROCF-CC appear to favor large wounds in which fibrin and/or a layer of devitalized tissue are still present. These characteristics may be observed in pressure ulcers, venous leg ulcers, surgical wounds, or diabetic foot wounds. The unique combination of NPWTi-d with ROCF-CC to promote granulation tissue formation and help remove devitalised tissue opens up a wide spectrum of clinical indications for the system, including situations where removing the devitalized tissue layers of undermined areas or large cavities is indicated. Table 1 displays an abbreviated list of wound types and characteristics that may benefit from use of NPWTi-d with ROCF-CC versus NPWT, based on our experiences with the foam at our institution.
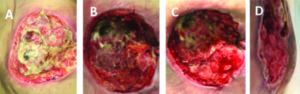 Figure 3: Buttock abscess at presentation (A). Most of slough removed on day 3 (B). Granulation tissue on day 6 (C). Granulation tissue formation on Day 9 (D).
Figure 3: Buttock abscess at presentation (A). Most of slough removed on day 3 (B). Granulation tissue on day 6 (C). Granulation tissue formation on Day 9 (D).
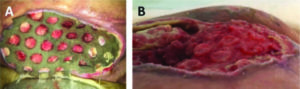 Figure 2: Columns of granulation tissue growing through the holes in the wound contact layer (A). Side angle showing depth of columns after dressing was removed on day 9 after initiation of NPWTi-d with ROCF-CC (B).
Figure 2: Columns of granulation tissue growing through the holes in the wound contact layer (A). Side angle showing depth of columns after dressing was removed on day 9 after initiation of NPWTi-d with ROCF-CC (B).
In certain situations such as a small epidermal wound combined with a large subcutaneous abscess, cavity or haematoma, surgical trimming remains indicated as a first-line approach in order to allow complete access of the foam dressing to the edges of the wound.
CONCLUSION
Surgical debridement has been defined as a clinical necessity to remove adherent, dead or contaminated tissue from a wound to facilitate the functional process of tissue However, constraints persist for a variety of reasons, including limited access to operating rooms, risks of anaesthesia, lack of clinical training, contraindications and specific underlying comorbidities of the patient. Confronted with these limitations, clinicians may opt for a conservative treatment approach with surgery as the default option. NPWTi-d using ROCF-CC may address some of these limitations and clinical needs, providing an alternative wound cleansing tool to be used when surgical debridement is not appropriate or is not accessible. The new ROCF-CC
 Figure 6: The wound contact layer with through holes was partially removed from the sacral pressure ulcer (A). The central portion of this ulcer was deeper than the peripheral portion (B). Careful removal of the remaining ROCF-CC dressing over the deepest part of the wound (C). Upon dressing removal, slough and nonviable tissue adhered to the portion of the wound contact layer placed in the deepest part of the wound (D).
Figure 6: The wound contact layer with through holes was partially removed from the sacral pressure ulcer (A). The central portion of this ulcer was deeper than the peripheral portion (B). Careful removal of the remaining ROCF-CC dressing over the deepest part of the wound (C). Upon dressing removal, slough and nonviable tissue adhered to the portion of the wound contact layer placed in the deepest part of the wound (D).
dressing appears to be a clinical step forward for NPWTi-d, but its effectiveness should be confirmed by further clinical studies and randomized controlled trials.
References
1.Strohal R, Dissemond J, O’Brien JJ, Piaggesi A, Rimdeika R, Young T, et al. EWMA document: debridement. An updated overview and clarification of the principle role of debridement. J Wound Care2013;22(Suppl)
2.Brinkert D, Ali M, Naud M, Maire N, Trial C, Teot L. Negative pressure wound therapy with saline instillation: 131 patient case series. Int Wound J 2013;10(Suppl 1):56 – 60.
3.Bollero D, Degano K, Gangemi EN, Aloj D, Malvasio V, Stella M. Long-term follow-up of negative pressure wound therapy with instillation: a limb salvage procedure? Int Wound J. 2016 Oct;13(5):768-73.
4.Fluieraru S, Bekara F, Naud M, Herlin C, Faure C, Trial C, 1 et al. Sterile-water negative pressure instillation therapy for complex wounds and NPWT failures. J Wound Care 2013; 22:293 – 9.
5.Gabriel A, Kahn K, Karmy-Jones R. Use of negative pressure wound therapy with automated, volumetric instillation for the treatment of extremity and trunk wounds: clinical outcomes and potential cost-effectiveness. Eplasty. 2014 Nov 3;14:e41.
6.Goss SG, Schwartz JA, Facchin F, Avdagic E, Gendics C, Lantis JC 2nd. Negative Pressure Wound Therapy With Instillation (NPWTi) Better Reduces Post-debridement Bioburden in Chronically Infected Lower Extremity Wounds Than NPWT Alone. J Am Coll Clin Wound Spec. 2014 Feb 20;4(4):74-80.
7.Kim PJ, Attinger CE, Oliver N, Garwood C, Evans KK, Steinberg JS, et al. Comparison of outcomes for normal saline and an antiseptic solution for negative-pressure wound therapy with instillation. Plast Reconstr Surg 2015;136:657e – 64e.
8.Téot L, Boissiere F, Fluieraru S. Novel foam dressing using negative pressure 14 wound therapy with instillation to remove thick exudate. Int Wound J 2017; 15doi: 10.1111/iwj.12719 16
9.Wolvos T. Wound instillation–the next step in negative pressure wound therapy. Lessons learned from initial experiences. Ostomy Wound Manage. 2004 Nov;50(11):56-66.
10.Wolvos T. The evolution of negative pressure wound therapy: negative pressure wound therapy with instillation. J Wound Care2015;24(Suppl 4b):15 – 20.
11.Gupta S, Gabriel A, Lantis J, Teot L. Clinical recommendations and practical guide for negative pressure wound therapy with instillation. Int Wound J 2016;13:159 –74.
12.Kim PJ, Attinger CE, Steinberg JS, Evans KK, Lehner B, Willy C, et al. Negative-pressure wound therapy with instillation: International Consensus Guidelines. Plast Reconstr Surg 2013;132:1569 – 79.
13.Kim PJ, AttingerCE, CristBD, GabrielA, GalianoRD, GuptaS,etal. Negative pressure wound therapy with instillation: review of evidence and recommendations. Wounds 2015:1 – 20.