
Dr. Traynor graduated from The Pennsylvania State University and received his doctor of podiatric medicine degree from The California School of Podiatric Medicine at Samuel Merritt University. Dr. Traynor is trained in foot and ankle trauma and reconstruction, elective surgery and wound care. He started his career in private practice in San Francisco, and has previously been involved with wound care centers in San Francisco and Daly City, CA. He now spends his time and at Highland Hospital, a level 1 trauma center in Oakland, California. Dr. Traynor is an Adjunct Assistant Professor for The California School of Podiatric Medicine and past Residency Director of the St. Mary’s Podiatric Surgery Residency Program. He is board certified by the American Board of Podiatric Medicine. Dr. Traynor has lectured on foot and ankle wound care and amputation prevention, is published in international orthopedic and podiatric surgical textbooks and journals on the treatment of the foot and ankle.
Traynor_Current Dialogues in Wound Management_2019_Volume 5_Issue 1
INTRODUCTION
Negative pressure wound therapy (NPWT) has been used clinically for almost three decades in the management of both acute and chronic wounds. Traditional electrically powered NPWT has been used by physicians and clinicians for the large, complex and heavily draining wound. Wounds created by large sacral defects, abdominal wall reconstruction and open amputations are commonly managed in part by NPWT. Over the past several years there has been a plethora of literature focusing on the diabetic ulcer with only a small percentage looking at the use of NPWT for treatment of diabetic foot ulcers (DFU). One reason for this is that the typical DFU isn’t particularly large in surface area or depth and tends to only be mild-to-moderate in drainage. That is not to say that larger, heavily draining DFU’s do not exist, but that is not the typical presentation. It has been my observation that we see less utilization of NPWT for the management of DFU’s even though we are familiar with the data surrounding the morbidity and mortality related to the diabetic foot ulcer in addition to the overall system costs associated with its treatment.
Over the course of my career, it has dawned on me that some providers may have forgotten how beneficial NPWT may be in this wound setting and, more importantly, that it is much more than just a drainage management system. With the use of NPWT, we are removing fluid and infectious materials from the wound bed, as well as promoting the development of granulation tissue. Additionally, we see the benefits of micro and macro strain when NPWT is used with reticulated open cell foam (ROCF) aiding in wound closure.1 In my experience, these properties are desirable throughout wound healing even though as the wound becomes small, the traditional NPWT can become cumbersome and time consuming specifically in terms of obtaining and maintaining a seal. The introduction of disposable mechanically powered NPWT (SNAP™ Therapy System) has made it easier to treat DFU’s with NPWT providing those same benefits to the wound bed seen with the traditional electrically powered NPWT.
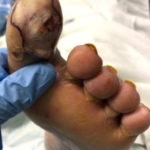 Figure 1. Wound within 24 hours of admission, prior to surgical prepa-ration for incision and drainage.
Figure 1. Wound within 24 hours of admission, prior to surgical prepa-ration for incision and drainage.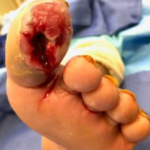 Figure 2. Wound after incision, drainage, and debridement. Following debridement, wound showed exposure of the long flexor and joint capsule.
Figure 2. Wound after incision, drainage, and debridement. Following debridement, wound showed exposure of the long flexor and joint capsule.
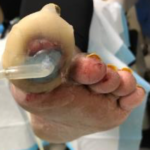 Figure 3. Initial appli-cation of the SNAP™ System, (7 days post-dis-charge).
Figure 3. Initial appli-cation of the SNAP™ System, (7 days post-dis-charge).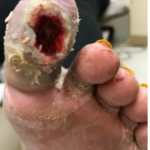 Figure 4. Wound after 4 days of SNAP™ System use.
Figure 4. Wound after 4 days of SNAP™ System use.
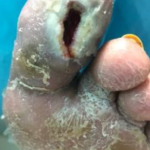 Figure 5. Wound after 2 weeks (4 applications) of SNAP™ System use.
Figure 5. Wound after 2 weeks (4 applications) of SNAP™ System use.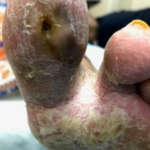 Figure 6. Wound less than 5 weeks from initiating SNAP™ System use. Patient no longer using the SNAP™ System which had been discontinued the week prior.
Figure 6. Wound less than 5 weeks from initiating SNAP™ System use. Patient no longer using the SNAP™ System which had been discontinued the week prior.
CASE REPORT
A 28-year-old female with non-insulin dependent diabetes mellitus and clinical obesity presented to the emergency room with concern for a local infection to her left great toe. The patient had no prior history of infection or ulceration. Upon examination, her vitals were stable, with symptoms limited to localized erythema, and edema of the hallux accompanied with pain. The erythema did not extend beyond the toe and no obvious abscess formation was seen. The patient was evaluated by the emergency department and discharged home with oral antibiotics. The patient returned to the emergency department 6 days later with a worsening infection, and abscess formation. She was admitted for intravenous (IV) antibiotics and taken to the operating room within 24 hours of admission for incision and drainage with debridement of all non-viable and grossly infected tissue (Figure 1). While consenting the patient for surgery, the risk of amputation was discussed due to the likely extent of the infection and non-viable tissue. Frank pus was evacuated from the hallux and localized necrosed tissue was excised down to the level of the long flexor tendon and joint capsule, the hallux interphalangeal joint had been spared. As part of our standard of care for grossly infected diabetic ulcers, pulse lavage post debridement with 3 L of 0.9% sodium chloride was initiated Post debridement, the wound was packed in the operating room (OR) with saline moist gauze (Figure 2). The patient was readmitted to the acute floor and the following day, NPWT using V.A.C.® Therapy was initiated. The advantage of waiting 24 hours between initial OR debridement and application of NPWT is that a quick assessment can be made in regard to the success of the debridement and current control of the infection. While it is not wrong to apply NPWT immediately, we find that it is wasteful while adding time to the OR and having to change the NPWT dressing 24 hours later without much added benefit in order to properly asses the wound. This patient’s wound was, of course, very difficult to seal based on its size and location. The V.A.C.® GRANUFOAM™ Dressing was then changed 2 days later (post-operative day 3). Due to the difficulty of application, the decision was made to discharge the patient home with saline wet-to-moist dressing and to continue with follow-up that week for alternative wound therapy. The patient’s antibiotic course was driven by OR cultures and she was discharged with a prescription for oral antibiotics. Subsequently, the patient was seen in clinic and the wound appeared stable upon assessment with no residual signs of infection 7 days after discharge, so the SNAP™ System was used (Figure 3). The system allowed for a much easier application than the inpatient V.A.C.® Therapy System.
So why use NPWT at all? The wound was not heavily draining nor did it have a large in surface area; it was, however, a deep wound down to the long flexor tendon and joint capsule with little-to-no granulation tissue noted. In our experience, this is a wound that would benefit most from NPWT. This is a wound with a chance of recurrence of infection and/or worsening of the wound that could result in amputation. While a hallux amputation may not constitute a major amputation and might be considered by many a success in limb salvage, the impact on the quality life for a 28-year-old female could be significant. The SNAP™ System was used in combination with off-loading that consisted of a controlled, ankle motion (CAM) walking boot with additional custom felt padding to off-load the wound and port that was placed directly over the wound. The patient was observed ambulating prior to discharge from clinic with the modified boot to ensure that the hallux was floating and not contacting the boot. The first dressing change was four days later at which one could see the wound base was almost 100% granular (Figure 4). The patient was seen twice weekly for dressing changes with the SNAP™ System, with weekly wound debridements. The wound continued to decrease in size with each application over the course of four weeks (Figures 5 and 6). The NPWT was stopped once the wound was too small for NPWT, showed with no depth, and had a surface area smaller than the port. The patient’s wound was fully healed at post-operative week 10 (Figure 7).
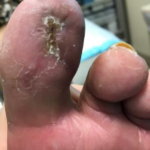 Figure 7. Wound fully healed 10 weeks post-surgical incision and drainage.
Figure 7. Wound fully healed 10 weeks post-surgical incision and drainage.
DISCUSSION
Disposable NPWT has been utilized sparingly in the U.S. for over 10 years. While traditional electrically powered NPWT has a proven track record with large complex wounds, NPWT has not been consistently used for smaller wounds. We need to remind clinicians that smaller, complex wounds, such as DFU’s, can still benefit from NPWT. With the development of the SNAP™ System, low exudating wounds and wounds smaller than 18 cm x 18cm can receive NPWT with patient outcomes similar to the outcomes achieved with traditional NPWT.5 While the patient presented in this case did not meet the typical description of a DFU patient, the risk of amputation was still high. The outcome of this case was successful due to patient education, off-loading, use of NPWT, activity modification, diet, and glucose control. While we had many options for advanced wound care, it is our opinion that none of them would have created granulation tissue and reduced the wound depth as reliably as NPWT.
References
1. Saxena V, Hwang CW, Huang S, Eichbaum Q, Ingber D, Orgill DP. Vacuum-assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg 2004;114:1086-1096.
2.Armstrong DG, Marston WA, Reyzelman AM, Kirsner RS. Comparative effectiveness of mechanically and electrically powered negative pressure wound therapy devices: A multicenter randomized controlled trial. Wound Repair Regen 2012;20:332-341.
3.Armstrong DG, Marston WA, Reyzelman AM, Kirsner RS. Comparison of negative pressure wound therapy with an ultraportable mechanically powered device vs. traditional electrically powered device for the treatment of chronic lower extremity ulcers: A multicenter randomized-controlled trial. Wound Repair Regen 2011;19:173-180.
4.Fong KD, Hu D, Eichstadt S et al. The SNaP system: biomechanical and animal model testing of a novel ultraportable negative-pressure wound therapy system. Plast Reconstr Surg 2010;125:1362-1371.
5.Tettelbach W, Cazzell S, Sigal F et al. A multicentre prospective randomised controlled comparative parallel study of dehydrated human umbilical cord (EpiCord) allograft for the treatment of diabetic foot ulcers. Int Wound J 2019;16:122-130.
Photos and patient information courtesy of Colin Traynor, DPM, St. Mary’s Medical Center, San Francisco, CA.
As with any case study, the results and outcomes should not be interpreted as a guarantee or warranty of similar results. Individual results may vary depending on the patient’s circumstances and condition.
NOTE: Specific indications, contraindications, warnings, precautions and safety information may exist for KCI products and therapies. Please consult a healthcare provider and product instructions for use prior to application. Rx only.

