
Dr. Subhas Gupta is the Chairman of the Department of Plastic Surgery at Loma Linda University in Southern California where he holds the academic rank of Professor and is the director of research. He is an innovative plastic surgeon who holds a PhD in Medical Informatics focused on the introduction of new technologies to clinical care. He is the director of the Loma Linda University Soft Tissue Reconstruction Team and directs a bio-investigative cell biology and wound healing laboratory.
Gupta_Current Dialogues in Wound Management_2020_Article_7
INTRODUCTION
The goals of wound reconstruction are well established and well accepted by wound care practitioners. These include the primary goal of closing the wound as well as secondary goals of preventing infection, providing stable and robust coverage, maximizing the function of the wounded region, and minimizing morbidity to any potential donor site as well as to the patient as a whole. These goals guide reconstructive philosophy and the planning that begins with wound bed preparation.
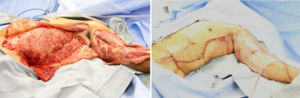
Wound bed preparation begins with the diagnosis of a wound and is inclusive of all interventions that optimize both the patient and the wound for subsequent closure. Often this is quite simple, as in the case of primary closure of an acute wound of the hand in an otherwise healthy patient. Wound bed preparation in these cases includes irrigation and debridement of any foreign matter before performing closure. Conversely, wound bed preparation of a trunk-based necrotizing infection in a diabetic patient with tobacco use, peripheral artery disease, and post-transplant immunosuppression is a far more complex process with multiple pathways ultimately leading to wound reconstruction (Figure 1). In a previous article, we presented a practical evolution of the reconstructive ladder that applies across care settings.1 This article outlined the wound and patient assessments necessary to select a reconstructive surgical technique.
RECONSTRUCTIVE LADDER EVOLUTION
As a paradigm integrated into the culture of reconstructive surgery, the reconstructive ladder has created a continually evolving framework which serves as a guide for wound closure and the range of options available to do so. With a history that stems from the central theme of a simple wound closure ladder initially described within the Edwin Smyth Surgical Papyrus,2 the reconstructive ladder application has evolved with time. In 600 BCE, wound closure moved to reconstruction, beginning with ear lobes and transposed flaps for nasal reconstruction in India (Figure 2). Advancement flaps later arose in Rome between 25 BCE and 50 CE, progressing to the use of skin grafts in the early nineteenth century under the work of Harold Gillies for facial reconstruction after World War I.3 As increasingly complex surgical procedures and technical innovations arose, the preservation of form and function remained fundamental to the decision-making of the reconstructive surgeon.

The traditional reconstructive ladder increases in complexity as one ascends beginning with secondary intention, direct tissue closure, skin grafts, and tissue transfer from local, distant, or free sites. The constant evolution of medical knowledge and improvement in surgical techniques has created an expansion of the reconstructive ladder, including several references to it as an elevator, clockwork, or matrix.4-6 Advanced therapies leading to this growth include tissue engineering, tissue expansion, negative pressure wound therapy, hyperbaric oxygen, cytokine-mediated therapy, and xeno/allograft skin substitutes or alternatives. The technical expertise of these surgical methods can be taught and learned by skilled surgeons, but when choosing which option to use, linear thought processes are no longer adequate. Optimal reconstructive strategies must maximize the patient benefit of form and functionality while minimizing morbidity and mortality.6 Consideration of patient risk factors and safety requires an evidenced-based approach that further provides the clinician with a modernized holistic reconstructive pathway. This holistic reconstructive pathway is patient-based, and geared towards favorable outcomes, improved patient quality of life, and economic sustainability.1
Guiding Wound Bed Preparation
The same assessments that guide our choice along the reconstructive ladder guide our wound bed preparation. Preparing a wound bed for closure must consider patient factors, wound physiology and wound anatomy. These three areas guide the five main parts of wound bed preparation that are found in Table 1.

Assessing a wound using the three categories in Table 1 in the context of a reconstructive goal from the holistic reconstructive ladder, allows the wound care practitioner to optimize each arm of wound bed preparation. Additionally, the consideration of each individual component in parallel allows for the creation of an ideal treatment plan that is efficient and cost-effective.
PATIENT OPTIMIZATION
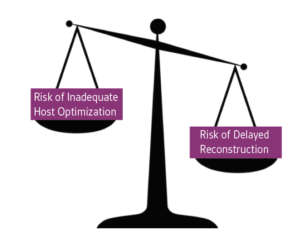
Patient preparation is the most obvious component of wound bed preparation but may be the most challenging as many wound healing comorbidities require long-term, sustained lifestyle and pharmaceutical changes. The clinician must decide when a host is optimized adequately to proceed with reconstruction. While investigators have postulated ideal levels of biochemical markers such as hemoglobin A1C and transthyretin, these levels must always be balanced in the context of the risk posed to the patient in continuing to have an open wound with the attendant risks of infection and other morbidities (Figure 3).
DEBRIDEMENT
Keeping in mind that wound bed preparation should be performed in all five categories in parallel, debridement is the next parameter to complete. There are five types of debridement including:7

Each one of these has a unique role to play in wound bed preparation, depending upon the wound physiology and, more importantly, the wound anatomy. Selecting the correct choice for debridement is generally not the ideal strategy as most wounds benefit from multiple modalities. The anatomic wound depth, along with surface characteristics, determine which method(s) should be used.8
Autolytic debridement uses the patient’s enzymes and moisture to break down the wound surface of scabs and eschar. This method, however, addresses only hypoxic and ischemic tissue and requires maintenance of a wound environment employing wound fluids to be in constant contact with the wound surface. This moist environment is attained by using semi-occlusive or occlusive dressings such as transparent films, hydrogels, and hydrocolloids. This method is most effective on wounds that are not heavily exudative. Autolysis is a selective process for necrotic tissue and is easy to execute, very effective, and painless for the patient. Unfortunately, this process is slow, and the wound must be carefully monitored for signs of infection and especially anaerobic growth that can occur under an occlusive dressing.8
Enzymatic debridement uses topically applied agents to break down necrotic tissue. This method is most useful for debriding wounds with a large amount of necrotic tissue or eschar formation. It works much faster than autolytic debridement with minimal risk to surrounding healthy tissue. A secondary dressing may be required to manage exudate and protect adjacent intact skin. Enzymatic debridement may be uncomfortable for the patient.8
Biologic debridement with maggots has been used for centuries but underwent a resurgence in use as bacterial resistance to antibiotics has increased wound infection rates. The current thinking around the mechanism of action of maggot biologic debridement is based on the idea that maggot excretions and secretions that contain proteolytic enzymes can digest necrotic, inflamed wound bed surfaces. Additionally, biologic debridement may be active on biofilms rendering this method of debridement useful for wounds with exposed hardware.7
Mechanical debridement remains a useful method of debridement for patients whose wounds have large amounts of necrotic tissue and who aren’t ideal candidates for surgical debridement. It uses the progression of wet-to-moist dressings that are removed at a predetermined frequency. These dressing changes result in a non-selective removal of the attached necrotic tissue wound surface, along with new granulation tissue. While this method is simple and cost-effective as only gauze and saline are used, it may remove healthy tissue as well as devitalized tissue on the surface. It can also be painful for the patient as it progresses.9
Surgical debridement uses sharp tools to remove necrotic tissue, either at a patient’s bedside or in an operating room under anesthesia. This method is best for large wounds with a vast amount of necrotic and infected material. This method allows for complete control over which tissue is removed, and it is the fastest way to achieve an improved wound bed. It is also the most resource-intensive and costly.9
EXUDATE MANAGEMENT
Wound exudate is produced naturally during the healing process. However, under- and over-production of wound fluid may delay wound healing. Exudate provides the essential moist wound environment and enables the movement of immune modifiers, growth factors, and tissue regenerating cells across the wound bed.10 Managing exudate in wound bed preparation is dependent upon the wound surface, the amount of exudate, and the concomitant debridement occurring as the wound is prepared for reconstruction. Wounds with significant necrotic tissue and eschar present are frequently dry and benefit from non-adhesive dressings along with hydrogels as debridement is occurring. Wounds with significant infectious material, such as slough and thick exudate, may benefit from negative pressure wound therapy (NPWT) with instillation (NPWTi-d). Wounds with hardened slough and higher amounts of exudate require more aggressive means of debridement prior to use of absorptive dressings or NPWT. Similarly, granulating wounds with higher amounts of exudate require fluid management with absorption or NPWT, but care must be taken to protect the regenerating tissue on the wound surface.
PERFUSION ENHANCEMENT
The next component of wound bed preparation for reconstruction involves assessing and optimizing the blood flow and tissue oxygenation of the wound bed. Wound tissues are dependent on the peripheral vasculature for oxygen and nutrient delivery. Macro- or microvascular delivery limitations may produce tissue hypoxia or ischemia that may prevent wound healing. Assessing tissue oxygenation involves non-invasive and invasive vascular studies alongside clinical examination and medical history. Surgical revascularization procedures may be necessary to increase tissue oxygenation.11 Local maneuvers such as edema control and lymphatic management are potentially critical along with topical modalities such as NPWT.
BIOBURDEN CONTROL
Bacterial bioburden in wounds is a principal contributor to inflammation, clinical wound infection, and impaired wound healing. Typical infection responses such as pain, erythema, calor, leukocytosis, edema, and increased wound exudate and wound odor, are often atypical in patients with chronic wounds due to the presence of comorbidities such as diabetes, neuropathy, and ischemia. Clinical examination assists in assessing bioburden, and while optimizing the wound bed, concurrent interventions to prevent infection are sometimes necessary. Controlling bioburden will likely reduce chronic inflammation and reduce the subsequent risk of wound infection both before and after reconstruction. Converting or advancing a wound from the inflammatory to the fibroplastic phase requires a reduction of bioburden. In addition to debridement and enhancing perfusion, topical modalities to reduce bacterial contamination may be helpful, including topical antimicrobials such as silver products. Additionaly, wound cleansing using NPWTi-d in conjunction with oral or intravenous antibiotics can help manage wound bioburden.12
CONCLUSION
Wound bed preparation indeed does start at the finish line by using the ultimate goal of the reconstructive ladder to guide the five components of patient optimization, debridement, exudate management, perfusion enhancement, and bioburden control. Careful, repeated examinations of the patient will yield important signals about wound physiology as it changes throughout treatment and help determine the time for reconstruction.
Photos courtesy of Dr. Gupta.
References
Gupta SC. A practical evolution of the reconstructive ladder across care settings: developing a value-based reconstructive pathway. Current Dialogues in Wound Management 2019;5(3):5-7.
Breasted JH. The Edwin Smith Surgical Papyrus. Chicago, IL: University of Chicago Press; 1930.
Gottlieb LJ, Krieger LM. From the reconstructive ladder to the reconstructive elevator. Plast Reconstr Surg 1994;93(7):1503-1504. doi:10.1097/00006534-199406000-00027.
Janis JE, Kwon RK, Attinger CE. The new reconstructive ladder: modifications to the traditional model. Plast Reconstr Surg 2011;127(Suppl 1):205S-212S.
Knobloch K, Vogt PM. The reconstructive clockwork of the twenty-first century: an extension of the concept of the reconstructive ladder and reconstructive elevator. Plast Reconstr Surg 2010;126(4):220e-222e. doi:10.1097/PRS.0b013e3181ec1eef.
Erba P, Ogawa R, Vyas R, Orgill DP. The reconstructive matrix: a new paradigm in reconstructive plastic surgery. Plast Reconstr Surg 2010;126(2):492-498.
Gottrup F, Jorgensen B. Maggot debridement: an alternative method for debridement. Eplasty 2011;11:e33.
Sieggreen MY, Maklebust J. Debridement: choices and challenges. Adv Wound Care 1997;10(2):32-37.
Gottrup F. Wound Debridement. In: Shukla VK, Mani R, Teot L, Pradhan S, eds. Management of Wound Healing. 1st ed. New Dehli, India: Jaypee Brothers Medical Publishers; 2007:107-119.
World Union of Wound Healing Societies. Consensus Document. Wound Exudate: effective assessment and management. London, UK: Wounds International; 2019.
Woo KY, Brandys TM, Marin JA. Assessing chronic wound perfusion in the lower extremity: current and emerging approaches. Chronic Wound Care Management and Research 2015;2:149-157.
Gabriel A, Heinrich C, Shores JT, Baqai WK, Rogers FR, Gupta S. Reducing bacterial bioburden in infected wounds with vacuum assisted closure and a new silver
dressing—a pilot study. Wounds 2006;18(9):245-255.
©Copyright 2020 3M. All rights reserved. 3M and the other marks shown are marks and/or registered marks. Unauthorized use prohibited. PRA-PM-US-02471 (05/20).

