
Ms. Milne is an Advanced Practice WOC Nurse providing care to patients across the continuum in acute care, long-term care, home health and outpatient settings. Employed by Connecticut Clinical Nursing Associates, she also provides consulting to organizations wishing to improve wound outcomes, conducts clinical research, lectures nationally and internationally, is the Co-Editor of the text Wound, Ostomy, Continence Secrets and provides clinical support to Wound Reference.com. She currently serves as the Co-chair of the Post-Acute Care Symposium. Additionally, she serves as a Nurse Board Member of the Association for the Advancement of Wound Care and an Associate Clinical Professor at the Yale School of Nursing. Ms. Milne is a paid consultant for 3M.
Milne_Current-Dialogues-in-Wound-Management_2023_Article-2
Twenty thousand newly diagnosed venous leg ulcers (VLUs) occur every year1 with 0.5%2 of the adult population in the U.S. having an active open wound daily. Patients will often present with a VLU to a provider who does not specialize in wound management. A lack of understanding related to the complexities of wound management in this patient population may lead to suboptimal outcomes.
The toll of VLUs is costly to the patient due to altered or loss of employment coupled with medical costs, strained family relationships, reduced socialization, and sequelae often related to pain management that includes constipation, falls, altered sleep patterns, and mentation changes.3
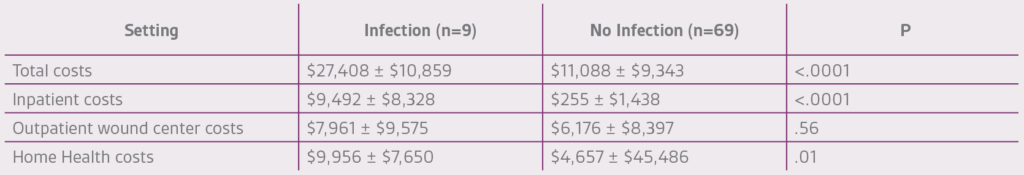
Costs for the management of VLUs can range from $150 million4 to $3 billion5 annually in the U.S. Individual episode expenditures vary by setting, though the literature is sparse in the skilled nursing facility/short term rehabilitation arena. It is no surprise that VLUs associated with wound infection add to this (Table 1). Also of note, patients with congestive heart failure are more likely to have a VLU-associated wound infection.4
A typical transitional scenario involves an acute hospitalization for a venous ulceration, complicated by cellulitis. The patient is usually managed by the hospitalist and local dressings are applied. The patient is then either discharged to a short-term rehabilitation setting or managed by a home health agency. The use of compression, the mainstay of treatment, is not well established. While a doppler ultrasound to rule out deep vein thrombosis is often done in this setting, it is rare to see vascular studies using an Ankle-Brachial Index, that can help determine the amount of appropriate compression the patient will need to assist in their wound healing.
Once the patient transitions to the new setting, a new provider assumes care. In the home setting, the primary care provider (PCP) is typically managing the patient with the home health care agency performing active wound care. If the patient is also followed at a local wound center, the home health nurse must take these recommendations, send them to the PCP who then approves, modifies, or rejects the recommendations. Each scenario is heterogeneous and may delay care as compression is not often initiated at the time of hospital discharge. Organizational formularies may prevent optimal compression or topical wound management choices. Additionally, provider skill, staff turnover, and a variety of payor reimbursement requirements add layers of complexity for patients with multiple comorbidities. The scenario is similar for patients transitioning from a short-term rehabilitation facility setting to a home health setting with a significant impact on the patient’s care plan. In my clinical experience, the key to transitioning lies in understanding disease etiology, the transition setting strengths and weaknesses, and patient engagement.
Understanding Disease Etiology
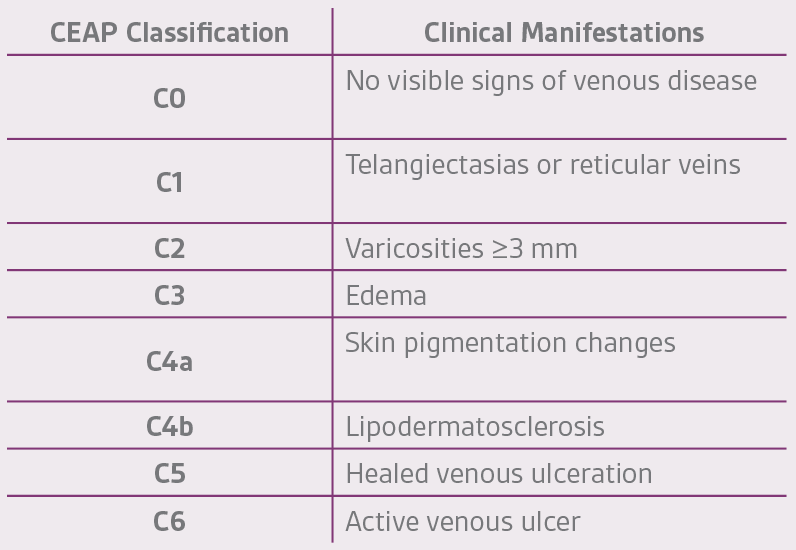
Venous disease severity is categorized by the CEAP (Clinical Manifestations, Etiology, Anatomic Distributions and Pathophysiology) system (Table 2) and clinical practice guidelines for management outlined by the Society of Vascular Surgery® and the American Venous Forum, which provide a structure to manage patients.
In humans, venous return is accomplished by a system of superficial veins connected to the deep veins by perforator veins. Through obstructive or reflexive mechanisms, weakness develops in the vein wall that results in venous disease. Varicosities typically form in the greater and lesser saphenous veins. Obstruction of the iliac veins or inferior vena cava can result in extensive varicose veins.5
Venous hypertension, venous valvular incompetence, and structural changes in the vein wall with associated inflammation are the primary pathophysiological mechanisms causing venous disease. Venous reflux disease is caused by venous valvular incompetence, venous outflow obstruction, or calf-muscle pump failure. Valvular incompetence occurs in either or both the superficial or deep venous system and results in venous hypertension below the affected valve. In patients with perforator vein incompetence, high pressure generated in the deep veins during calf muscle contraction is transmitted to the superficial system. Valvular incompetence results from structural changes of the valve leaflets. A combination of microvascular and macrovascular events culminates into visible ulcerations, though the precise pathophysiological process has not been specifically elucidated.5
The vein wall weakening, and subsequent dilatation contributes to disease progression. On the microvascular side, histological studies have demonstrated disrupted smooth muscle cell and elastin arrangement, overproduction of collagen Type I with concomitant reduction of collagen Type III and abnormal deposition of fibroblast and transforming growth factors β within the vein wall. Overproduction of collagen Type I decreased synthesis of collagen Type III, and disruption of the arrangement of smooth muscle cells and elastin fibers have been observed in histological studies of varicose venous segments.2 Increased levels of tissue inhibitors of matrix metalloproteinases observed in varicose vein specimens may favor the deposition of extracellular matrix material in the vein wall. Increased levels of transforming growth factor β1 and fibroblast growth factor β have also been observed in the walls of varicose veins and may contribute to structural degradation.6
When dilatation occurs, the stretched vein leaks fibrinogen, growth factors and white blood cells into the surrounding interstitial space, subcutaneous tissue, and dermis. Pathophysiological hypotheses suggest that the formation of fibrin cuffs surround the vessels leading to the loss of oxygen and nutrient diffusion, an inflammatory cascade is initiated with the trapping of leukocytes in the area, and growth factors imperative for healing are absent, as they are physically bound within the exudate contents that have escaped from the dilated vein.7 Loss of the calf muscle pump contributes to venous engorgement, as calf muscle dysfunction affects venous function. Absent or reduced calf muscle strength alters venous return hemodynamics causing blood to pool in the lower extremity and adding to capillary leak and subsequent edema.8
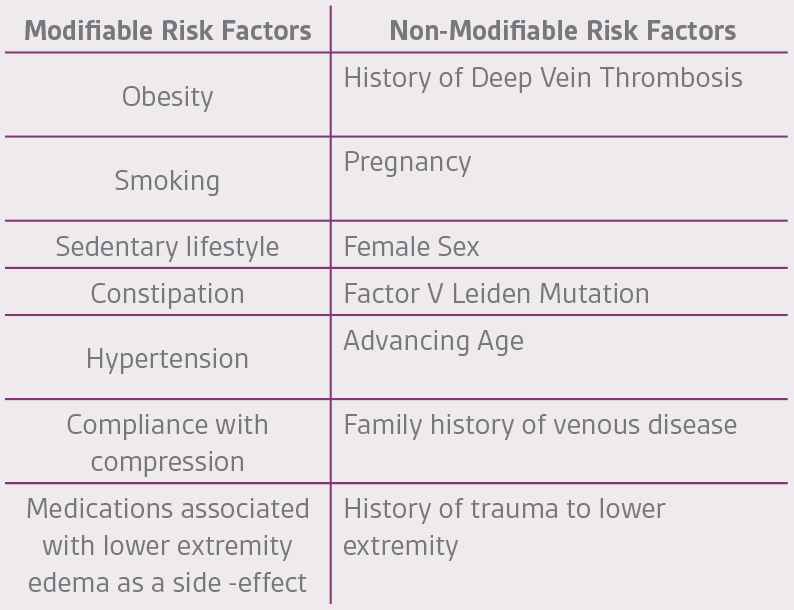
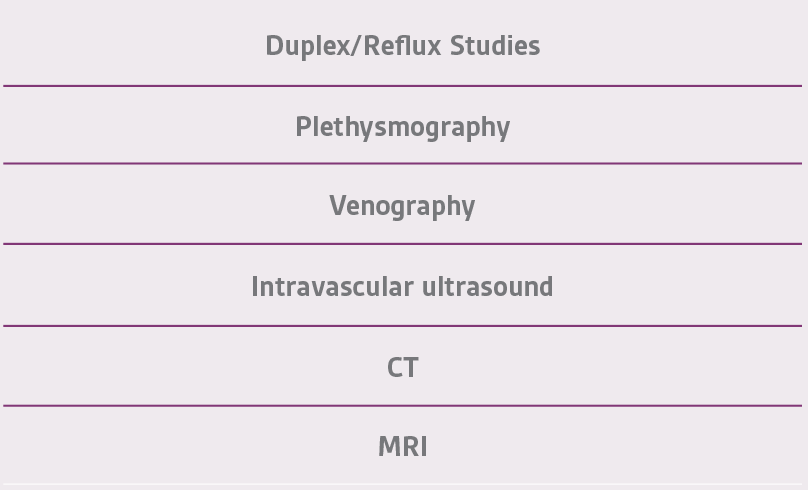
As in the management of any wound, eliminating the underlying cause is critical in achieving successful outcomes. Determining the root cause – obstruction, reflux, or both – is of utmost importance along with evaluation of the calf muscle pump. Risk factors (Table 3), modifiable and non-modifiable, combined with the physical signs commonly associated with venous insufficiency (Table 4) are first determined followed by diagnostic testing (Table 5).
courtesy of Catherine T. Milne, MSN, APRN, ANP/ACNS-BC, CWOCN-AP

courtesy of Catherine T. Milne, MSN, APRN, ANP/ACNS-BC, CWOCN-AP
While waiting for test results, a simple vascular exam with an ankle-brachial index and toe pressure, if available, can assist to determine the amount of compression applied at the first visit. It is important to note that even in a patient with established arterial disease, edema management must be addressed, as edema alone will impair arterial function. Mild compression in this subset of patients is recommended.9 Referring the patient to a skilled vascular surgeon who can correct the underlying obstructive or reflexive disorder should be considered. The wound specialist should manage the highly exudative and often inflamed wound coordinating care with direct caregivers in the setting that the patient spends most of their time in order to optimize outcomes.
Identifying Strengths and Weaknesses in the Transition Setting
Direct communication with caregivers helps clarify patient goals, identify care issues, and allows for providers to expediently alter the plan of care as needed to optimize outcomes. Common barriers include a lack of knowledge in the application of multilayered compression, the substitution of a multilayered compression wrap to a zinc-based impregnated gauze wrap (Unna Boot), knowledge about wound exudate and periwound skin management, and an underappreciation of bioburden and the role of inflammation in wound healing. Identifying key caregivers and presenting information in a collaborative manner can help promote knowledge transfer thereby allowing the wound provider to learn about organizational barriers in the transitioned setting that may prevent optimal outcomes. Ongoing and frequent conversations with caregivers improve team dynamics and often serve as a “safety huddle”.10
Case Study #1:
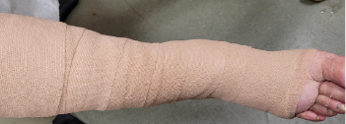
An 89-year old female in a skilled nursing facility with a positive family history for venous disease, long-standing bilateral leg edema with hemosiderin deposition and stasis dermatitis, sustained a traumatic wound when transferring to her wheelchair (Figures 1-3). Multiple therapies were attempted but wound healing ultimately failed. The in-house nurse practitioner asked for a wound consultation.
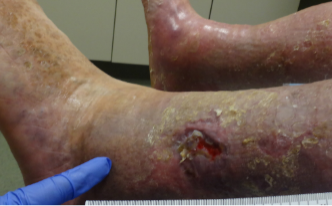
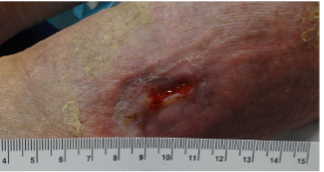
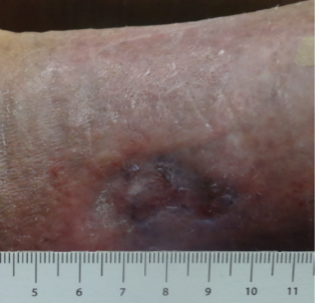
Patient Engagement
VLUs are associated with recurrence rates of 60%-70% over a ten-year period11 making it critical that providers actively engage the patient in the management of their disease at the initial visit. Explaining the pathophysiology, and treatment strategies while communicating that the chronicity of the disease is manageable over the course of the patient’s lifetime is imperative. Assessing the patient’s ability for engagement is the first step and is well outlined in the literature.12 While patients vary in their ability to participate in the care of their VLU, this should not result in those caring for the patient to discontinue encouragement and education about ongoing disease management. By asking patients to articulate perceived barriers in disease management, and specifically addressing these issues, one can nurture the patient’s involvement in their own care.
Case Study #2:
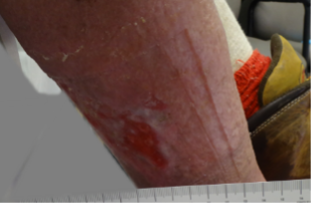
A 69-year-old morbidly obese male with a 10-month history of venous disease presented at our facility (Figures 4-6). The patient had been managed by his primary care provider (PCP) in his home with the assistance of a home health nurse, who changed his dressings three times per week with a calcium alginate and gauze wrap. No compression was used. The patient reported his biggest problem was that he wet his socks and bed sheets and could not wear shoes. The patient also reported that he sat in his kitchen chair all day and did not elevate his legs because he was concerned that he might ruin his furniture. An ankle brachial index of 0.99 with triphasic wave forms was noted. The patient did not wish to pursue diagnostic testing due to stigma associated with his symptomology.
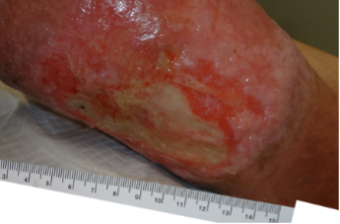
Catherine T. Milne, MSN, APRN, ANP/ACNS-BC, CWOCN-AP
Summary
VLUs are a challenging wound management issue. Correcting the pathophysiological abnormality, when possible, in combination with the use of an evidence-based local wound management and compression strategy, as well as an appreciation of the strengths and constraints of the management setting can help optimize patient outcomes.
References
- Gloviczki P, Comerota AJ, Dalsing MC, et al. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011;53(5 Suppl):2S-48S. doi:10.1016/j.jvs.2011.01.079
- Davies AH. The Seriousness of Chronic Venous Disease: A Review of Real-World Evidence. Adv Ther. 2019;36(Suppl 1):5-12. doi:10.1007/s12325-019-0881-7
- Chamanga ET. Understanding venous leg ulcers. Br J Community Nurs. 2018;23(Sup9):S6-S15. doi:10.12968/bjcn.2018.23.Sup9.S6
- Melikian R, O’Donnell TF Jr, Iafrati M. The economic impact of infection requiring hospitalization on venous leg ulcers. J Vasc Surg Venous Lymphat Disord. 2022;10(1):96-101. doi:10.1016/j.jvsv.2021.06.012
- McArdle M, Hernandez-Vila EA. Management of Chronic Venous Disease. Tex Heart Inst J. 2017;44(5):347-349. doi:10.14503/THIJ-17-6357
- Piazza G. Varicose veins. Circulation. 2014;130(7):582-587. doi:10.1161/CIRCULATIONAHA.113.008331
- Çerman AA, Altunay IK, Karabay EA. Venous Leg Ulceration. In: Alexandrescu V, ed. Wound Healing-New insights into Ancient Challenges. London: IntechOpen; 2016. DOI: 10.5772/63962. Accessed October, 17, 2022.
- Orr L, Klement KA, McCrossin L, et al. A Systematic Review and Meta-analysis of Exercise Intervention for the Treatment of Calf Muscle Pump Impairment in Individuals with Chronic Venous Insufficiency. Ostomy Wound Manage. 2017;63(8):30-43. doi:10.25270/owm.2017.08.3043
- Wound, Ostomy and Continence Nurses Society. Venous, arterial, and neuropathic lower-extremity wounds: Clinical resource guide. Mt. Laurel, NJ: Wound, Ostomy and Continence Nurses Society; 2019
- Titler MG. The Evidence for Evidence-Based Practice Implementation. In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Rockville (MD): Agency for Healthcare Research and Quality (US); April 2008.
- Kolluri R, Lugli M, Villalba L, et al. An estimate of the economic burden of venous leg ulcers associated with deep venous disease. Vasc Med. 2022;27(1):63-72. doi:10.1177/1358863X211028298
- Moore Z. Kapp S, Looney A, et al. A tool to promote patient and informal carer involvement for shared wound care. Wounds Intl. 2021;12(3):86-92.
NOTE: Specific indications, contraindications, warnings, precautions, and safety information exist for these products and therapies, some of which may be Rx only. Please consult a clinician and product instructions for use prior to application.
As with any case study, the results and outcomes should not be interpreted as a guarantee or warranty of similar results. Individual results may vary depending on the patient’s circumstances and condition.
Patient data and images courtesy of Catherine T. Milne MSN, APRN, ANP/ACNS-BC, CWOCN-AP
©2023 3M. All rights reserved. 3M and the other marks shown are marks and/or registered marks. Unauthorized use prohibited. 3M marks used under license in Canada. All other marks are property of their respective owners.



