
Elizabeth Faust is a Nurse Practitioner in Wound, Ostomy, and Continence Care at the Reading Hospital in West Reading, PA. She graduated from Gwynedd Mercy University with her MSN in Adult Nurse Practitioner in 2009, became a Certified Wound Specialist (CWS) in 2010, and certified in Wound, Ostomy, and Continence (CWOCN) in 2012. She serves as an in-patient wound, ostomy, and continence specialist for a 700+-bed Level I Trauma Center. She focuses on care of the perioperative, post-operative, cardiac and critical care patients, with an interest in nursing and physician education and V.A.C. VERAFLO™ Therapy.
Ms. Faust is a paid consultant for 3M
Faust_Current-Dialogues-in-Wound-Management_2023_Article-5
Negative pressure wound therapy (NPWT) utilizes negative pressure to draw wound edges together, remove exudate and infectious materials, and promote the development of granulation tissue. However, advances in technology since the beginning of its use in the United States (US) in 1995 have led to the development of multiple therapeutic modalities inducing open abdomen negative pressure therapy and incisional negative pressure therapy. Recently, traditional NPWT has evolved to include a therapy phase with instillation and dwell time of topical wound solutions (3M™ Veraflo™ Therapy), allowing for the addition of wound cleansing capabilities to traditional NPWT. The dressings for each of these modalities have also been updated resulting in a full toolkit of NPWT products that can be utilized based upon the unique needs of the patient and the wound characteristics.
It is well-known that final wound closure should not occur until the wound bed is properly prepared.1-3 Options for final wound closure often include the use of flaps or grafts (allograft, xenograft, or autograft). Previously, traditional NPWT has been used pre- and post-grafting in complex wounds to assist with closure; however, Veraflo Therapy can also assist with wound bed preparation.
Dressing options with Veraflo Therapy
To highlight the innovation of the newest dressing option for Veraflo Therapy, it is important to review what has been available historically. Automated Veraflo Therapy first came to market in 2011 with two foam dressing options, one drape, and two tubing sets. The foam dressing, which is less hydrophobic than the 3M™ V.A.C.® Granufoam™ Dressing but has a similar composition, is available in a black version (3M™ V.A.C. Veraflo™ Dressing) or a grey tubular version (3M™ V.A.C. Veraflo Cleanse™ Dressing). The V.A.C. Veraflo Cleanse Dressing has a higher tensile strength and is intended for use in wounds with complex geometries. The drape that comes with these dressings has adhesive properties more suited for instillation therapy than traditional NPWT as well. The tubing set options include a single pad (3M™ V.A.C. VeraT.R.A.C.™ Pad) or a dual pad (3M™ V.A.C. VeraT.R.A.C. Duo™ Tube Set). They consist of two tubes, one for instillation and one for NPWT. In the single pad option, the tubes are on the same port, in the dual pad option, they are separate.
In 2015, a novel foam was released in the US market. This foam dressing was similar in composition to the V.A.C. Veraflo Cleanse Dressing but had three pieces of the grey foam: one contact layer with through holes, and two options for a cover layer (3M™ V.A.C. Veraflo Cleanse Choice™ Dressing). The purpose of the contact layer is to facilitate the removal of thick exudate, while the cover layers are used based upon wound depth for continuous NPWT coverage over the holes. Initial clinical research performed in France showed a reduction in non-viable tissue in the wound base with use of Veraflo Therapy and V.A.C. Veraflo Cleanse Choice Dressing in complex wounds.2
In 2019, a hybrid drape consisting of a combination of acrylic and silicone-based adhesives (3M™ Dermatac™ Drape) was released in the US market. When used with traditional NPWT, Dermatac Drape helped reduce patient reported pain scores during drape removal across 10 clinical sites and 39 drape removals.4 Use of Dermatac Drape with Veraflo Therapy has been shown to maintain a negative pressure and instillation seal without periwound skin irritation and helped reduce patient reported pain levels during dressing changes.5-9 Initially, Dermatac Drape was offered separately, but now is also offered with V.A.C. Therapy dressing kits.
Latest Innovation in Veraflo Therapy Dressings
A new dressing option came to market in 2022 for Veraflo Therapy. This new dressing is a combination of the thin cover layer and the wound contact layer with through holes of the V.A.C. Veraflo Cleanse Choice Dressing fused as a single piece of blue double-sided foam dressing (3M™ Veraflo™ Cleanse Choice Complete™ Dressing). The Veraflo Cleanse Choice Complete Dressing can be purchased as a dressing kit (3M™ Veraflo™ Cleanse Choice Complete™ Dressing Kit), which includes Dermatac Drape. The V.A.C. VeraT.R.A.C. Pad provided with the dressings are dependent upon the dressing size, with the large sized dressing coming with the dual tubing set option. The goal of the Veraflo Cleanse Choice Complete Dressing Kit is to provide the clinician with one complete set of dressings, drape, and tubing that can suit the needs of patients who are being considered for Veraflo Therapy. If removal of thick or fibrinous exudate is warranted, then wound care clinicians can simply put the side with through holes down into the wound bed. If not, clinicians may use the flat side.
Initial Experience with Veraflo Cleanse Choice Complete Dressing
We utilized Veraflo Therapy with Veraflo Cleanse Choice Complete Dressings in three patients with traumatic lower extremity (LE) wounds for wound bed preparation. In all three cases, the patient suffered a crush injury. These types of injuries may not declare themselves for weeks, require extensive surgery, and typically involve larger, more complex wounds. The following treatment protocol was utilized to manage these traumatic LE wounds:
Excisional surgical debridement for non-viable tissue
Application of Veraflo Therapy using V.A.C. Veraflo Cleanse Choice Complete Dressing with the instillation of a hypochlorous solution using a 10-minute dwell time, followed by 2 hours of continuous -125 mmHg negative pressure wound therapy
Xenograft application (engineered extracellular matrix [EECM]) with traditional NPWT used as a bolster
Split Thickness Skin Graft
Once the wound bed was properly prepared with the use of surgical debridement and Veraflo Therapy with Veraflo Cleanse Choice Complete Dressings, patients were able to receive an EECM that allowed for transition to the next level of care. In our experience, this wound management protocol ultimately decreased patient length of hospital stay, operating room visits, and time to closure, compared to previous protocols utilizing either traditional NPWT or standard of care dressings at our institution. Overall, the use of Veraflo Therapy with the Veraflo Cleanse Choice Complete Dressing and EECM had a positive impact on complex soft tissue injury management. Representative case images depicting wound bed improvement with Veraflo Therapy and V.A.C. Veraflo Cleanse Choice Complete Dressings are shown in Figure 1.
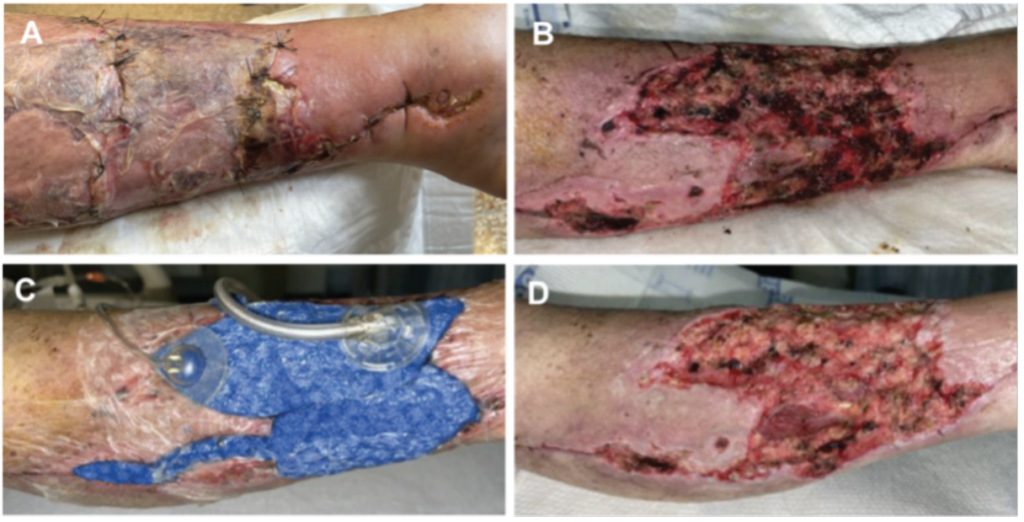
Discussion
Initial experiences using Veraflo Therapy with Veraflo Cleanse Choice Complete Dressings for management of traumatic injuries reported improved wound bed preparation for all 3 patients. In my clinical experience, the stepwise approach of utilizing surgical debridement, Veraflo Therapy with 3M™ Veraflo™ Cleanse Choice Complete™ Dressing, dermal grafting with a NPWT bolster, and final closure with a split-thickness skin graft has helped facilitate acute wound care resulting in reduction of operating room visits, earlier patient discharge, and ultimately decreased time to closure. Based on this, the trauma department at our institution has adopted the use of Veraflo Therapy with the Veraflo Cleanse Choice Complete Dressing in the reconstructive ladder as described above. Our institution is now utilizing this therapy for a variety of wound etiologies and wound locations. Given the health economic literature around the use of Veraflo Therapy, health economic evaluations for Veraflo Therapy with the Veraflo Cleanse Choice Complete Dressings should be investigated.
References
- Kim PJ, Lookess S, Bongards C, Griffin LP, Gabriel A. Economic model to estimate cost of negative pressure wound therapy with instillation vs control therapies for hospitalised patients in the United States, Germany, and United Kingdom. Int Wound J. 2022;19(4):888-894. doi:10.1111/iwj.13689
- Teot L, Boissiere F, Fluieraru S. Novel foam dressing using negative pressure wound therapy with instillation to remove thick exudate. Int Wound J. 2017;14(5):842-848. doi:10.1111/iwj.12719
- Societies WUoWH. Strategies to reduce practice variation in wound assessment and management: The T.I.M.E. Clinical Decission Support Tool. Report Consensus Document. 2020 Sep 10. Accessed 2022 Nov 28. https://www.woundsinternational.com/resources/details/strategies-reduce-practice-variation-wound-assessment-and-management-time-clinical-decision-support-tool
- Kharkar P, Napolitano RJ, Jr., Lantis J, et al. Assessment of a Novel Drape Containing Acrylic and Silicone-based Adhesives When Using Negative Pressure Wound Therapy. In: DR. 2019:
- Fernandez LG, Matthews MR, Benton C, et al. Outcomes with use of novel silicone-acrylic hybrid drape during negative pressure wound therapy with fluid instillation: initial clinical experience. In: DR.
- Obst MA. Novel use of a hybrid silicone-acrylic drape with negative pressure wound therapy with instillation. In: DR.
- Greenstein E. Use of negative pressure wound therapy with instillation and a novel silicone hybrid drape: an initial experience. In: DR.
- Napolitano RJ, Jr., Gittins ME, Laub S. Negative pressure wound therapy with instillation after total knee arthroplasty: initial use of a novel silicone-acrylic drape. In: DR.
- Desvigne MN. Negative pressure wound therapy with instillation and a novel silicone hybrid drape use in complex wounds. In: DR.
NOTE: Specific indications, contraindications, warnings, precautions and safety information exist for these products and therapies. Please consult a clinicians and product Instructions for Use prior to application. Rx only.
As with any case study, the results and outcomes should not be interpreted as a guarantee or warranty of similar results. Individual results may vary depending on the patient’s circumstances and condition.
Patient data and images courtesy of Elizabeth Faust, MSN, CRNP, CSWS, CWOCN-AP, MAPWCA
©2023 3M. All rights reserved. 3M and the other marks shown are marks and/or registered marks. Unauthorized use prohibited. Used under license in Canada.

