
Dr. Graham graduated with a baccalaureate degree in Biomedical Sciences from Grand Valley State University in 2009. He then went on to attain his Doctorate of Podiatric Medicine in 2014 from Barry University.Dr. Graham is currently in his 1st year of podiatric medicine and surgical residency with the added credential of reconstructive rearfoot/ankle surgical training (PMSR/RRA) at Jackson North Medical Center in Miami, Florida. He also serves as the president of the Podiatry Overseas Association, a non-profit medical mission organization.
Graham_Current Dialogues in Wound Management_2015_Volume 1_Issue 3
Protein lysis plays several essential roles in the natural healing process of a wound.1Two major families of proteases including more than 20 enzymes are involved in the wound healing process. Initially low levels of proteolytic enzymes are involved in ridding the wound environment of bacteria (note that the level of proteolytic activity is important since if the enzyme is not active it is dormant or not capable of functioning); overlapping hemodynamic processes including the formation and degradation of the fibrin clot, stimulation of cellular migration including inflammatory cells, keratinocytes, and fibroblasts, and mitigation of selected growth factor activity (PDGF and TGF-β1) represent the mainstays of the acute wound healing model.
A wound entering a period of chronicity, ranging by definition from 2 to 4 weeks, becomes “stuck” in the inflammatory phase of healing.3, 4 Prolonged inflammation fosters a noxious wound environment5, primarily caused by elevated protease activity (i.e. matrix metalloproteases [MMPs] and serine elastase), making wound healing challenging. In a prospective study by Serena involving 162 patients, a chronic wound with elevated protease activity had a 90% probability of not healing without protease-altering intervention.
Laboratory testing for protease activityinclude gelatin zymography, substrate based assays and enzyme-linked immunosorbent assays (ELISA), among others.7In vitro research utilizing chronic wound fluid has consistently revealed elevated protease activity and quantity, most notably MMPs 2, 8, 9, and neutrophil-derived elastase (serine elastase).8-10 The predominant cell in the inflammatory phase of wound healing remains the neutrophil; this cell type is responsible for the production of serine elastase, which explains why this enzyme is prevalent in the chronic wound. Conversely, acute wounds were noted to have low protease activityfostering a normal, well-orchestrated healing cascade and thus a greater healing potential.
Many studies have documented elevated levels of MMPs in chronic nonhealing wounds, with decreased levels of tissue inhibitor of metalloproteases (TIMPs).11-13 TIMPs are responsible for down-regulating the over activity of MMPs. With fewer TIMPs present to deactivate these enzymes, the MMPs are in abundance and allowed to create and maintain an inflamed chronic wound environment.
Wysocki et al14 determined that MMP-2 and MMP-9 are elevated in chronic wound fluid by factors of five to 10 times, compared to acute wounds. In this study, acute postoperative mastectomy wounds were compared with chronic leg ulcerations. Wound fluid samples showed elevated active enzymes and proenzymes. Researchers concluded that elevated metalloproteinase activity levels could lead to a high tissue turnover rate, which could lead to failure of wound closure (Figure 1).
Nwomeh et al9 evaluated structural protein collagen and its degradation in the wound healing process, as collagenase represents the rate-limiting step in the breakdown of the extracellular matrix (ECM). This research compared tissue and fluid samples in acute and chronic ulcers. In chronic ulcerations, MMP-8 was consistently the predominant collagen-degrading proteolytic enzyme. Healing nonchronic wound samples showed MMP-8 almost exclusively in its inactive form. Chronic nonhealing wound samples showed significant levels of active MMP-8, which represents high tissue turnover rate and destruction of the collagen-based ECM.
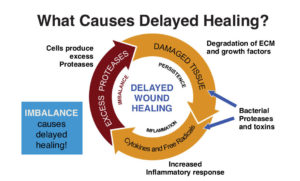 Figure 1. The Cullen Circle describes how the hostile wound environment continues until an intervention such as collagen/ORC “breaks the cycle” – Ref: Snyder R. Point of care diagnostic tests
Figure 1. The Cullen Circle describes how the hostile wound environment continues until an intervention such as collagen/ORC “breaks the cycle” – Ref: Snyder R. Point of care diagnostic tests
Grinnel et al15 studied burn wounds and identified neutrophil elastase (serine elastase) to be the proteolytic enzyme responsible for fibronectin degradation. Fibronectin is an essential glycoprotein in the ECM and an early component in the formation of granulation tissue.16,17 Therefore, it is implied that wounds with elevated neutrophil elastase have decreased granulation tissue formation. Therefore, it is hypothesized that wounds with increased levels of neutrophil elastase have a decreased potential for healing. Additional studies show that there is a trend of increased neutrophil elastase activity in chronic wounds compared to acute wounds.
Left unchecked, increased proteolytic enzyme activity may completely degrade the ECM. This structure may be more than the collagen latticework it was once thought to be. Bornstein et al19 first coined the term “dynamic reciprocity” (DR) which describes a bidirectional interaction between the ECM and the cells that surround it. This theory essentially claims that the ECM is not just a framework to build upon, but a communication center within the healing wound. Schultz et al20 expanded upon the original DR research and proposed that within the DR model the ECM is responsible for sending signals that affect surrounding cellular migration, differentiation, and proliferation. Therefore, destruction of the ECM could decrease the ability of the ECM to communicate with the cells that surround it, thus creating delays in wound healing.
Wound care therapies which maintain an optimal moist wound environment can aid in lowering protease levels, while also being potentially efficient and cost effective; these include debridement21,22 among others. Oxidized regenerated cellulose (ORC)/ collagen matrix works by binding to MMPs and growth factors. ORC/collagen works by sequestering growth factors while binding to proteases and cleaving these enzymes, rendering them inactive. It is hypothesized that as the ORC/collagen matrix degrades, active growth factors are released back into the wound. ORC/collagen also helps create a physiologically moist wound environment conducive to granulation tissue formation and re-epithelialization. In a 12-week prospective study of 27 patients, Smeets et al23concluded that the use of ORC/collagen showed a significant and immediate reduction in protease activity in wound fluid samples taken from venous stasis ulcers compared to a control group receiving treatment with hydrocolloid dressings. Other studies show that the use of ORC/collagen with and without a silver component assists in healing patients in shorter time frames and is more cost effective when compared to the use of saline wet-to-dry dressings.
A natural and healthy amount of protease activity should exist in any wound; the negative effects of elevated protease activity begin when protease volume and activity overcomes the natural acute needs of healing. Irrespective of the treatment of choice, it is clear that restoring a chronic wound’s biochemical environment to resemble that of an acute wound is advantageous in wound healing. By decreasing excessive MMPs and serine elastase, we are providing a more nurturing wound healing environment.
References
1.Gibson D, Cullen B, Legerstee R, et al. MMPs made easy. Wounds Intl. 2009;1(1):1-6.
2.Snyder R, Cullen B. Point of care diagnostic tests in wound management: targeted therapy for excessive protease activity – the first frontier. Wound Care & Hyperbaric Medicine. 2011;2(3):59–67.
3.Chen SM, Ward SI, Olutoye OO, et al. Ability of chronic wound fluids to degrade peptide growth factors is associated with increased levels of elastase activity and diminished levels of proteinase inhibitors. Wound Repair Regen. 1997;5:23-32.
4.Falanga V, Grinnell F, Gilchrest B, et al. Workshop on the pathogenesis of chronic wounds. J Invest Dermatol. 1994;102:125-7.
5.Tarnuzzer RW, Schultz GS. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen. 2002;4(3):321-5.
6.Serena, T, Cullen B, Bayliff S, et al. Protease activity levels associated with healing status of chronic wounds. Poster presented at Symposium for Advanced Wound Care; September 12–14, 2012; Baltimore, MD.
7.International consensus. The role of proteases in wound diagnostics. An expert working group review. London: Wounds International 2011.
8.Trengove NJ, Stacey MC, MacAuley S, et al. Analysis of the acute and chronic wound environments: the role of protease and their inhibitors. Wound Repair Regen. 1999;7(6):442-52.
9.Nwomeh BC, Liang H-X, Cohen KI, et al. MMP-8 is the predominant collagenase in healing and non-healing ulcers. J Surg Res. 1999;81(2):189-95.
10.Cullen B, Smith R, McCulloch E, et al. Mechanism of action of PROMOGRAN, a protease modulating matrix for treatment of diabetic foot ulcers. Wound Repair Regen. 2002;10:16-25.
11.Beidler SK, Douillet CD, Berndt DF, et al. Multiplexed analysis of matrix metalloproteinases in leg ulcer tissue of patients with chronic venous insufficiency before and after compression
12.Subramaniam K, Pech CM, Stacey MC, et al. Induction of MMP-1, MMP-3 and TIMP-1 in normal dermal fibroblasts by chronic venous ulcer wound fluid. Int Wound J. 2008;5:79-86.
13.Lobmann T, Ambrosch A, Schultz G, et al. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia 2002;45:1011-6.
14.Wysocki AB, Staliano-Coico L, Grinnell F. Wound fluid from chronic leg ulcers contain elevated levels of metalloproteinases MMP-2 and MMP-9. J Invest Dermatol. 1993;101:64-8.
15.Grinnell F, Zhu M. Identification of neutrophil elastase as the proteinase in burn wound fluid responsible for degradation of fibronectin. J Invest Dermatol. 1994;103:155-61.
16.Pankov R, Yamada K. Fibronectin at a glance. J Cell Sc. 2002;115(Pt 20):3861–3.
17.Herrick S, Ashcroft G, Ireland G, et al. Up-regulation of elastase in acute wounds of healthy aged humans and chronic venous leg ulcers are associated with matrix degradation. Lab Invest. 1997;77:281−8.
18.Trengove NJ, Stacey MC, MacAuley S, et al. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen. 1999;7:442–52.
19.Bornstein P, McPherson J, Sage H. Synthesis and secretion of structural macromolecules by endothelial cells in culture. In: Nossel JL, Vogel HJ, eds. Pathobiology of the endothelial cell. New York, NY: Academic Press; 1982:215-28.
20.Schultz GS, Davidson JM, Kirsner RS, et al. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen.2011;19:134–48.
21.Stechmiller JK, Kilpadi DV, Childress B, et al. Effect of vacuum-assisted closure therapy on the expression of cytokines and proteases in wound fluid of adults with pressure ulcers. Wound Repair Regen. 2006;14(3):371–4.
22.Kilpadi D, Stechmiller JK, Childress B, et al. Composition of wound fluid from pressure ulcers treated with V.A.C.® Therapy in home-health or extended care patients. Wounds. 2006;18(5):119–26.
23.Smeets R, Ulrich D, Unglaub F et al. Effect of oxidised regenerated cellulose/collagen matrix on proteases in wound exudate of patients with chronic venous ulceration. Int Wound J. 2008;5:195–203.
24.Lázaro-Martínez JL, Aragón-Sánchez FJ, García-Morales E, et al. A retrospective analysis of the cost-effectiveness of a collagen/oxidized regenerated cellulose dressing in the treatment of neuropathic diabetic foot ulcers. Ostomy Wound Manage. 2010;56(11A):4–8.
25.Snyder RJ, Richter D, Hill ME. A retrospective study of sequential therapy with advanced wound care products versus saline gauze dressings: comparing healing and cost. Ostomy Wound Manage. 2010;56(11A):9–15.

