
Dr. Robert Klein completed podiatric medical school in Chicago at the Rosalind Franklin University of Medicine and Science, Scholl College of Podiatric Medicine. Dr. Klein continued his surgical training as the Chief Resident at Michigan Health Center in Detroit. He is a Clinical Assistant Professor in the Department of Surgery at the University of South Carolina School of Medicine (USCSOM) Greenville and specializes in wound care, limb preservation, and Dr. Klein is a paid consultant for 3M.
Klein_Current-Dialogues-in-Wound-Management_2022_Article-2
Negative pressure wound therapy (NPWT) has been used for over thirty years in the management of acute and chronic wounds. NPWT can be used in the acute hospital setting as well as after hospital discharge in multiple settings including skilled nursing facilities, rehabilitation hospitals, long-term acute hospitals, and in the home. Wounds that are commonly treated with NPWT include chronic, acute, traumatic, subacute, and dehisced wounds, partial-thickness burns, ulcers (such as diabetic, pressure, or venous insufficiency), flaps and grafts.1,2
Clinicians who frequently use NPWT are seeking new ways to improve the operational and clinical benefits of NPWT. Although highly effective in promoting wound healing, the current traditional acrylic adhesive drapes used with NPWT have some limitations which include:
- Pain experienced by the patient at dressing changes
- Difficulty in repositioning the drape once applied
- The time required for dressing changes
- The time required for training new or infrequent users
- Creating a seal in anatomically challenging areas
- The drape sticking to gloves
3M has developed a first-to-market novel hybrid drape (3M™ Dermatac™ Drape) consisting of a polyurethane film with acrylic adhesive and a silicone perforated layer for use with 3M™ V.A.C.® Therapy.3 Dermatac Drape, with its silicone/acrylic hybrid design, creates an ideal balance for wound healing. Some of the benefits of the Dermatac Drape include:
- Providing a highly effective seal for negative pressure
- Repositionability at the time of initial placement
- Less time at dressing changes compared to traditional acrylic-only adhesive drapes
- Minimizing pain and discomfort at dressing changes
Over the past year, our center participated in a pilot project using Dermatac Drape outside of the hospital setting. Five consecutive patients were enrolled in the pilot between September 2021 and December 2021. At baseline, all patients were receiving V.A.C.® Therapy using traditional acrylic adhesive drape technology. Upon discharge from the hospital and/or after evaluation at the wound care center, the patient’s NPWT drape was transitioned to Dermatac Drape. All patients received regular V.A.C.® Therapy dressing changes through their respective Home Health Agency (HHA) every 2-3 days. The patients were followed once a week in our wound care center to evaluate wound healing and assess the patient’s and the HHA’s impressions of Dermatac Drape. The wound types treated in this pilot included diabetic foot ulceration and acute surgical wounds (Figure 1). All patients went on to wound healing.
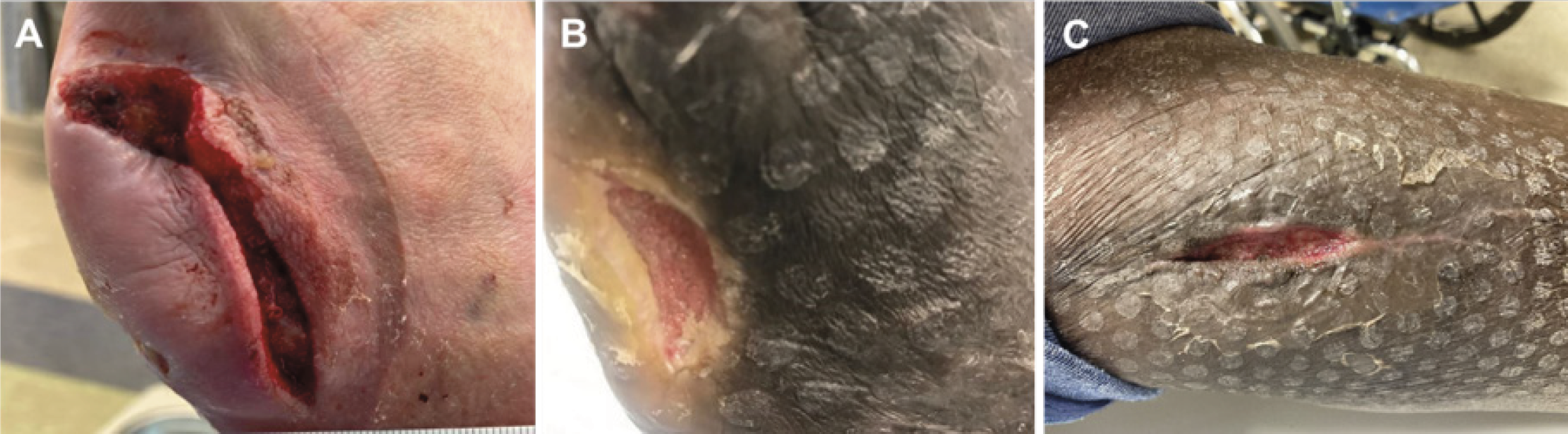
The physicians, patients, and the HHAs observed tangible benefits with the use of Dermatac Drape in comparison to the traditional acrylic adhesive drape. Observed benefits of Dermatac Drape included:
- Significant ease of use
- No need for skin preparation before applying the drape
- No need for the drape window framing technique to be used in order to achieve a seal
- Less cutting of the drape
- Use of a single clear release liner
- Minimal (if any) discomfort at wound during dressing changes
- Significantly less time required for dressing changes
As with any new product or technology there is a learning curve. It is recommended that clinicians and members of the wound care team participate in a hands-on demonstration and/or workshop prior to applying Dermatac Drape. It is also critical to keep in mind that patient and wound selection are important considerations in the use of Dermatac Drape, clinicians should review the instructions for use prior to application. Reaching out to experienced resources including your local representative or the 3M Helpline can be extremely helpful to answer any questions or troubleshoot any problems, if encountered.
Having used Dermatac Drape, our center can attest to the ease of use of applying the hybrid drape compared with the “1-2-blue” application technique for the traditional acrylic adhesive drape. The HHA nurses that participated in this pilot shared similar feedback around the ease-of-use with Dermatac Drape.
A few tips that should be kept in mind when applying the drape include:
- Leave a 5-7 cm drape border when applying Dermatac Drape (Figure 2)
- Cut slits in the Dermatac Drape to accommodate difficult anatomy
- The acrylic adhesive on Dermatac Drape can take up to 20 minutes after placement to cure, allowing for repositionability in this timeframe.

This small, single-center pilot using V.A.C.® Therapy with Dermatac Drape in the out of hospital setting indicates that this hybrid silicone/acrylic drape was easy to handle, cut, apply, and reposition. In addition, a number of HHA nursing staff reported reduction in the time required for dressing changes with the use of Dermatac Drape.
References
- McM1. Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997; 38: 563-576; discussion 577 [PMID: 9188971 DOI: 10.1097/00 000637-199706000-00002]
- Panayi AC, Leavitt T, Orgill DP. Evidence based review of negative pressure wound therapy. World J Dermatol 2017; 6(1): 1-16 Available from: URL: http://www.wjgnet.com/2218-6190/ full/v6/i1/1.htm DOI: http://dx.doi.org/10.5314/wjd.v6.i1.1
- Fernández LG, Matthews MR, Benton C, et al. Use of a novel silicone-acrylic drape with negative pressure wound therapy in anatomically challenging wounds. Int Wound J. 2020;17:1829–1834. https:// doi.org/10.1111/iwj.13471
Patient data and images courtesy of Robert Klein, DPM, FACFAS, CWS FFPM RCPS (Glasgow)
As with any case study, the results and outcomes should not be interpreted as a guarantee or warranty of similar results. Individual results may vary depending on the patient’s circumstances and condition.
NOTE: Specific indications, contraindications, warnings, precautions and safety information exist for these products and therapies, some of which may be Rx only. Please consulta a clinician and product instructions for use prior to application.
©2022 3M. All rights reserved. 3M and the other marks shown are marks and/or registered marks. Unauthorized use prohibited. Used under license in Canada.

