Dr. Fernandez is a Clinical Assistant Professor of Surgery and Family Practice at the University of Texas Health Center at Tyler. He is also an Attending Teaching Staff in the Division of Surgery for Mother Frances Health System and East Texas Medical Center in Tyler. Dr. Fernandez obtained his residency training at Loyola University, the University of Illinois, the University of Chicago and Northwestern University, Chicago, Illinois, with special training in Trauma and Burns at Kings County Hospital, SUNY Down State System, Brooklyn, New York, NY. He is well published in multiple areas of research both in peer-reviewed articles and book chapters, and has made numerous oral and poster presentations at national and international conferences.
Fernandez_Current Dialogues in Wound Management_2017_Volume 3_Issue 3
NOTE: As with any case study, the results and outcomes should not be interpreted as a guarantee or warranty of similar results. Individual results may vary depending on the patient’s circumstances and condition.
THE WHY
Complex and chronic wounds in the United States affect over 6 million patients, with nearly $50 billion (U.S.) spent on their treatment annually1, 2. The treatment of chronic wounds and the concomitant economic burden is growing rapidly due to increasing health care costs, an aging population and a sharp rise in the incidence of diabetes and obesity worldwide 3-5.
Complex wounds have a significant impact on patient outcomes, morbidity, mortality cost and lead to longer hospital stays 6, 7. As a result of the cumulative advances in medical care in the last century, an aging population, a sharp rise in the incidence of diabetes and obesity worldwide, and the subsequent improvement in survival, the incidence of complex and chronic wounds appears to be increasing8. It is, therefore, incumbent for surgeons to be well versed in all aspects of the care and management of patients with complex wounds, their co-morbidities, and to be skilled in the use of new technologies that can accelerate the wound’s repair process.
THE HOW
The proliferation of new strategies in prevention, treatment and management has improved the care of complex and chronic wounds patients significantly in the last few decades. The development of negative pressure wound therapy (NPWT), introduced commercially after the studies of Argenta and Morykwas in 1997, was a seminal turning point in the way we approached the care of the complex/chronic wound9. NPWT creates an environment that promotes wound healing by preparing the wound bed for closure, reducing edema, promoting granulation tissue formation and perfusion, and by removing exudate and infectious material. These mechanisms and other effects of NPWT have been validated by various experimental and clinical studies 9-17.
MECHANISMS OF ACTION
In their excellent review, Huang et al, noted that clinical observations into the effect of NPWT have identified 4 primary mechanisms of action: (1) the immediate decrease in size of the wound when negative pressure is applied termed macro deformation; (2) micro deformation: the effects at the foam-wound interface; (3) fluid and debris removal; and (4) stabilization of the wound environment (Figure 1)18-19.
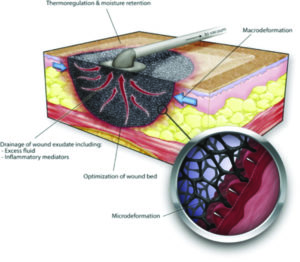 Figure 1: Huang C, Leavitt T, Bayer LR, Orgill DP. Effect of negative pressure wound therapy on wound healing. Curr Probl Surg. 2014;51(7):301-331.
Figure 1: Huang C, Leavitt T, Bayer LR, Orgill DP. Effect of negative pressure wound therapy on wound healing. Curr Probl Surg. 2014;51(7):301-331.
There are also secondary effects involved in direct mechanical sheer stretching of the cellular cytoskeleton. The microstrain or cell stretch created by the black reticulated open cell foam (ROCF-G; V.A.C.® GRANUFOAM™ Dressing) is hypothesized to be translated to the cells by cell surface receptors linked to the extracellular matrix (eg, integrins) (Figure 2)11 and may affect biological tissue responses, which can potentially impact wound healing. The microstrain can create tissue deformations, which may stimulate cellular activity and lead to granulation tissue formation.11, 18-20
In vivo evidence has shown that porcine non-infected full-thickness wounds treated with NPWT with instillation and dwell time (NPWTi-d; V.A.C. VERAFLO™ Therapy) using saline and a specialized reticulated open cell foam dressing (ROCF-V; V.A.C. VERAFLO™ Dressing) demonstrated increased granulation tissue formation compared to standard NPWT with ROCF-G dressing after 7 days of therapy.21, 22 They concluded that: “Negative pressure wound therapy with controlled instillation of fluids (NPWTi) is the next generation of
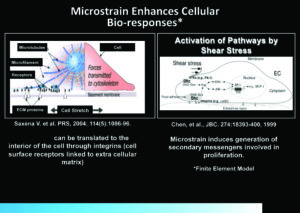 Figure 2: Modes of delivery of Negative Pressure Wound Therapy and their effects
Figure 2: Modes of delivery of Negative Pressure Wound Therapy and their effects
negative pressure therapy”, and that the new V.A.C.ULTA™ Therapy System and the specialized ROCF-V wound dressing provides discrete wound irrigation and fluid removal phases between negative pressure cycles and simplifies wound cleansing by allowing for automated and contained volumetric wound lavage. The new ROCF-V dressing has modified hydrophobic and mechanical properties compared to the existing ROCF-G dressing, enhancing fluid delivery while potentially reducing the likelihood of tearing or particle retention at dressing changes. This study also shows NPWTi-d with ROCF-V to be effective in a non-infected porcine wound model, resulting in more granulation tissue than standard NPWT with ROCF-G; the authors noted that these results should be confirmed in human studies 21-22.
NPWT can be delivered as continuous pressure or intermittent mode of pressure21. In their ex vivo study of porcine skin, Lessing et al, described the two types of intermittent modes of NPWT: “intermittent NPWT, where negative pressure alternates between a set pressure and no pressure for programmed periods of time; and dynamic (variable) NPWT, in which negative pressure transitions between a high pressure and a low pressure following programmed rise and fall times“ (Figure 321-22)
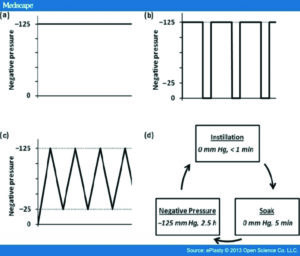 Figure 3: Lessing MC, James RB, Ingram SC. Comparison of the effects of different negative pressure wound therapy modes -continuous, noncontinuous, and with instillation – on porcine excisional wounds. Eplasty 2013; 13:e51.
Figure 3: Lessing MC, James RB, Ingram SC. Comparison of the effects of different negative pressure wound therapy modes -continuous, noncontinuous, and with instillation – on porcine excisional wounds. Eplasty 2013; 13:e51.
They also compared the different modes of therapy with negative pressure utilizing saline instillation. They noted that the average observed granulation thickness was not statistically different among the different modes of NPWT wounds at day 7. They did note, however, that the average granulation thickness of NPWTi-d treated wounds was statistically greater (P < .05) by 44%, 57%, and 40%, respectively, than that of wounds treated with continuous, intermittent, and dynamic NPWT. (Figure 4 21)
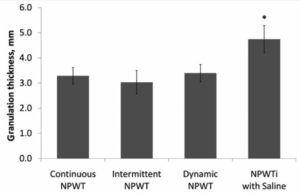 Figure 4: Measured granulation tissue thickness of treated wounds.
Figure 4: Measured granulation tissue thickness of treated wounds.
In clinical practice, the solutions used can vary from topical wound cleansers to antiseptics to normal saline. Gabriel et al demonstrated in a small study of 15 patients with complex infected wounds that instilling silver nitrate helped to reduce bioburden, decreased days of treatment, time to clearance of wound infection, time to wound closure and allowed early hospital discharge compared with the standard moist wound-care therapy control group patients23-26.
CURRENT USE AND RECOMMENDATIONS OF NPWTi-d
The use of NPWTi-d appears to be increasing, manifesting in a growing body of reported practical and comparative clinical literature27-30. Several best practice expert panel recommendations have been developed to better guide the clinician in the most appropriate use of NPWTi-d31, 32. When utilizing NPWTi-d, a topical wound solution is introduced into the wound bed and dwells for a planned period of time and then is removed during a cycle of NPWT. The therapy provides cleansing of the wound bed and removal of infectious materials. The existing literature shows NPWTi-d may be used to prepare the wound to enhance granulation tissue formation between operative debridements and may be utilized in various wound types. (Figure 518, 30-32)
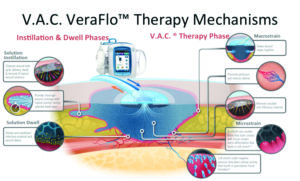 Figure 5: 1. Rycerz AM, et al. Int Wound J 2013;10:214-220. 2. Saxena SM, et al. Plast Recon Surg 2004;114(5):1086-1095. 3. McNulty AK, et al. Wound Repair Regen 2009 Mar;17(3):192-199. 4. McNulty AK, et al. Wound Repair Regen 2007;15:838-846.
Figure 5: 1. Rycerz AM, et al. Int Wound J 2013;10:214-220. 2. Saxena SM, et al. Plast Recon Surg 2004;114(5):1086-1095. 3. McNulty AK, et al. Wound Repair Regen 2009 Mar;17(3):192-199. 4. McNulty AK, et al. Wound Repair Regen 2007;15:838-846.
THE WHO:Case Presentations
Case 1: COMPLEX OPEN ABDOMEN
This is a female, in her late sixties who has a history of prior rue en y bariatric gastric by-pass 10 yrs. prior to her admission. The patient had undergone emergency abdominal exploration and is found to have ischemic bowel and undergoes resection of 54 cm of efferent jejunum, abdominal washout, and initial damage control surgery.
Postoperatively, the patient has multiple organ system failure abdominal compartment syndrome and was managed with the ABTHERA™ Open Abdomen Negative Pressure Therapy System for her open abdomen. After multiple abdominal washouts, the patient improves, undergoes successful intestinal re-anastomosis and is closed with STRATTICE™ Reconstructive Tissue Matrix (RTM; LifeCell Corporation, an Allergan Affiliate, Parsippany, NJ).
Approximately 2 weeks later, the patient presents with bloody-purulent discharge from the abdominal incision and partial
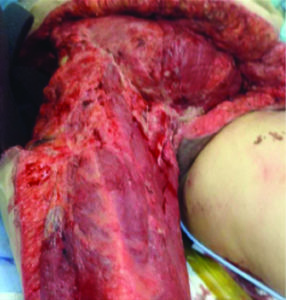
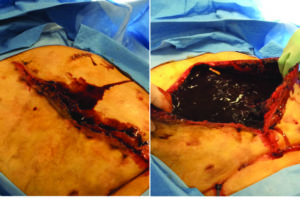 Figure 6: Patient with enteroatmospheric fistula, during initial debridement.
Figure 6: Patient with enteroatmospheric fistula, during initial debridement.
dissolution of the STRATTICE™ RTM due to an enteroatmospheric fistula (Figure 6, 7).
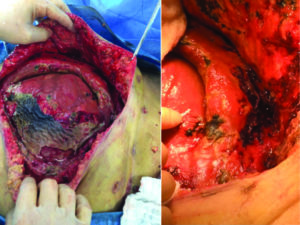 Figure 7: Patient with enteroatmospheric fistula, during initial debridement. The remnants of the STRATTICE™ RTM can be seen on the right image. The surgeons’ finger points to the enteroatmospheric fistula.
Figure 7: Patient with enteroatmospheric fistula, during initial debridement. The remnants of the STRATTICE™ RTM can be seen on the right image. The surgeons’ finger points to the enteroatmospheric fistula.
The underlying viscera were protected with the use of ADAPTIC TOUCH™ Non-Adhering Silicone Dressing and V.A.C. WHITEFOAM™ Dressing (polyvinyl alcohol) was used with V.A.C.® Therapy. An ostomy negative pressure ring was constructed to control the fistula. After the enteroatmospheric fistula was controlled, V.A.C.® Therapy was discontinued, and the patient underwent therapy with V.A.C. VERAFLO™ Therapy with saline instillation (dwell time of 10 minutes and NPWT for 2 hours) until an adequate granulation tissue bed was obtained (Figure 8, 9).
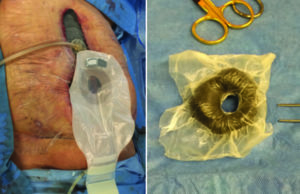 Figure 8: Patient with enteroatmospheric fistula, during initial debridement. The wound was debrided, the underlying tissue was protected with a layer of ADAPTIC TOUCH™ Dressing and V.A.C. WHITEFOAM™ Dressing. An ostomy negative pressure ring was constructed to control the fistula.
Figure 8: Patient with enteroatmospheric fistula, during initial debridement. The wound was debrided, the underlying tissue was protected with a layer of ADAPTIC TOUCH™ Dressing and V.A.C. WHITEFOAM™ Dressing. An ostomy negative pressure ring was constructed to control the fistula.
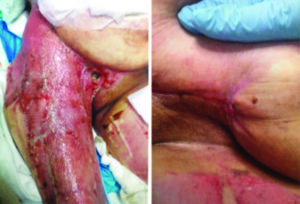
The patient was then transitioned to standard V.A.C.® Therapy. The patient achieved significant re-epithelialization and the enteroatmospheric small bowel intestinal fistula was well controlled. The patient was able to tolerate small frequent meals and meet her nutritional requirements. The patient underwent inpatient rehabilitation and was discharged home after a prolonged hospital/rehab stay (Figure 10).
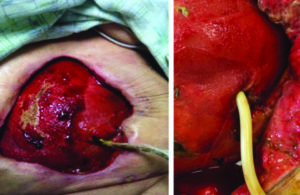 Figure 9: Excellent granulation tissue noted on a clean wound base after V.A.C. VERAFLO™ Therapy with saline. A Foley catheter was used to control fistula output.
Figure 9: Excellent granulation tissue noted on a clean wound base after V.A.C. VERAFLO™ Therapy with saline. A Foley catheter was used to control fistula output.
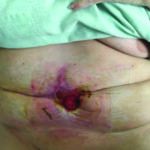 Figure 10: Excellent re- epithelialization of tissue without surgical flap advancement required.
Figure 10: Excellent re- epithelialization of tissue without surgical flap advancement required.
Case 2: NECROTIZING FASCIITIS
The patient is a 40-year-old female with a history of insulin dependent diabetes mellitus, hypertension, morbid obesity, adult respiratory distress syndrome, multiple organ system failure on pressors due to septic shock from massive perineal, and pelvic necrotizing fasciitis. The patient’s right thigh and perineal area is shown after initial debridement, subsequent second debridement and V.A.C. VERAFLO™ Therapy with saline (dwell time of 10 minutes and NPWT for 2 hours) on postoperative day 2-10 (Figure 11-13).
The patient underwent multiple debridements and subsequent transition to standard V.A.C.® Therapy. On hospital day 70, the patient underwent split-thickness skin grafting to the right thigh, inguinal area, perineum, supra-pubic area, right buttocks and back with application of ACell’s MatriStem®, MicroMatrix® 2-1000 mg EM and four 10 x 15 cm ACell’s MatriStem® wound sheets. The patient was transferred to an inpatient rehabilitation center and subsequently discharged home, tolerating a diet and ambulatory without the need for fecal diversion(Figure 14).
The use of V.A.C. VERAFLO™ Therapy with normal saline contributed to positive outcomes for both patients. These included reduction in anticipated morbidity, mortality, successful wound healing, and returning to the previous level of functioning. Despite consideration of amputation of the right limb and fecal diversion in the case of the second patient, both of these patients have returned to their preadmission level of activity.
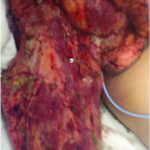 Figure 11: Necrotizing fasciitis patient s/p initial debridement and V.A.C. VERAFLO™ Therapy with saline, postoperative day 2.
Figure 11: Necrotizing fasciitis patient s/p initial debridement and V.A.C. VERAFLO™ Therapy with saline, postoperative day 2.
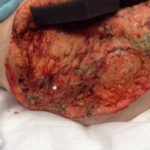 Figure 12: Necrotizing fasciitis patient s/p initial debridement and V.A.C. VERAFLO™ Therapy with saline, postoperative day 2.
Figure 12: Necrotizing fasciitis patient s/p initial debridement and V.A.C. VERAFLO™ Therapy with saline, postoperative day 2.
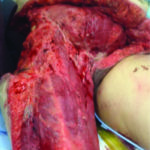 Figure 13: Necrotizing fasciitis patient s/p 2nd debridement and V.A.C. VERAFLO™ Therapy with saline.
Figure 13: Necrotizing fasciitis patient s/p 2nd debridement and V.A.C. VERAFLO™ Therapy with saline.
Figure 14: Definitive wound coverage with split thickness skin graft on HD 70.
CONCLUSION
Chronic and complex wounds continue to be a significant challenge in wound healing. Several factors, such as the patient’s comorbidities, topographic location of the wound, presence of fistulae, presence of fomites (e.g. synthetic vascular grafts, orthopedic-neurosurgical hardware), active infection and the chronicity of the tissue injury can cause the wound to deteriorate and stall.
Randomized, controlled clinical studies should be developed to compare the effectiveness of NPWTi-d to other, non-NPWT methods of care and to determine the effect of NPWTi-d versus standard irrigation practices on wound healing and over all patient outcomes. The optimal treatment paradigm in preparation of the wound bed should be that which is the most efficient in removing elevated inflammatory cytokines, enzymatic elements, cellular debris, necrotic tissue, and infection. Automated wound cleansing combined with negative pressure therapy is a revolutionary new tool in wound management. NPWTi-d has been shown to have a marked and positive effect in the cleansing of devitalized tissue, removal of exudates, debris, militating against bioburden, a more rapid creation of granulation tissue and effectively creating a healthier wound bed. As experience with this new approach expands, we will have better data that will help inform the clinician as to what therapeutic regimen is most appropriate for the treatment of the complex, chronic wound.
References
1.Fife CE, Carter MJ. Wound care outcomes and associated cost among patients treated in US outpatient wound centers: data from the US Wound Registry. Wounds 2012; 24:10–17 [PubMed] [Ref list]
2.Driver VR, et al. The costs of diabetic foot: the economic case for the limb salvage team. J Am Podiatr Med Assoc 2010; 100:335–341 [PubMed] [Ref list]
3.Hossain P, Kawar BEl, Nahas M. Obesity and diabetes in the developing world–a growing challenge. N Engl J Med. 2007; 356:213–5. [PubMed] [Ref list]
4.Administration on Aging A; Services, DoHaH. A profile of older Americancs. 2007. Pp. 1–19. http://www.wvseniorservices.gov/Portals/0/pdf/ProfileOfOlderAmericans.pdf. [Ref list]
5.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999; 341:738–46. [PubMed] [Ref list]
6.Ferreira MC, Tuma P Jr, Carvalho VF, Kamamoto F. Complex wounds. I (Sao Paulo). 2006;61(6):571-8.
7.Kirkland KB, Briggs JP, Trivette SL, et al. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999 Nov. 20(11): 725-30. [Medline].
8.Sen CK, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009; 17:763–771 [PMC free article] [PubMed]
9.Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997;38(6):563-76; discussion 577. [ Links ]
10.KCI Licensing. V.A.C. Therapy Clinical Guidelines. San Antonio, TX: Kinetic Concepts, Inc; 2010.
11.Saxena V, Hwang CW, Huang S, Eichbaum Q, Ingber D, Orgill DP. Vacuum-assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg. 2004; 114(5):1086–96; discussion 1097–8.
12.McNulty AK, Schmidt M, Feeley T, Kieswetter K. Effects of negative pressure wound therapy on fibroblast viability, chemotactic signaling, and proliferation in a provisional wound (fibrin) matrix. Wound Repair Regen. 2007; 15(6):838–46.
13.McNulty AK, Schmidt M, Feeley T, Villanueva P, Kieswetter K. Effects of negative pressure wound therapy on cellular energetics in fibroblasts grown in a provisional wound (fibrin) matrix. Wound Repair Regen. 2009; 17(2):192–9.
14.Scherer SS, Pietramaggiori G, Mathews JC, Prsa MJ, Huang S, Orgill DP. The mechanism of action of the vacuum-assisted closure device. Plast Reconstr Surg. 2008; 122(3): 786–97.
15.Dastouri P, Helm DL, Scherer SS, Pietramaggiori G, Younan G, Orgill DP. Waveform modulation of negative-pressure wound therapy in the murine model. Plast Reconstr Surg.2011; 127(4): 1460–6.
16.Armstrong DG, Lavery LA. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet. 2005; 366(9498): 1704–10.
17.Blume PA, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers. Diabetes Care. 2008;31(4):631–6.
18. Huang C, Leavitt T, Bayer LR, Orgill DP. Effect of negative pressure wound therapy on wound healing. Curr Probl Surg (2014) 51(7): 301–31. Doi: 10.1067/j.cpsurg.2014.04.001PubMed Abstract | CrossRef Full Text | Google Scholar
19.D.P. Orgill, E.K. Manders, B.E. Sumpio, et al.The mechanisms of action of vacuum assisted closure: more to learn. Surgery, 146 (1) (2009), pp. 40–51
20. Chen KD, Li YS, Kim M, Li S, Yuan S, Chien S, Shyy JY. (1999) J Biol Chem 274:18393-400.
21. Lessing C, Slack P, Hong KZ, Kilpadi D, McNulty A. Negative pressure wound therapy with controlled saline instillation (NPWTi): dressing properties and granulation response in vivo. Wounds. 2011; 23:309–19. [PubMed]
22. Lessing MC, James RB, Ingram SC. Comparison of the effects of different negative pressure wound therapy modes – continuous, noncontinuous, and with instillation – on porcine excisional wounds. Eplasty 2013; 13:e51.
23. Gabriel A, Shores J, Heinrich C, Bagai W, Kalina S, Sogioka N, et al. Negative pressure wound therapy with instillation: a pilot study describing a new method for treating infected wounds. Int Wound J.2008; 5:399–413. [PubMed]
24. Schintler MV, Prandl EC, Kreuzwirt G, Grohmann MR, Spendel S, Scharnagl E. The impact of the VAC Instill in severe soft tissue infections and necrotizing fasciitis. Infection. 2009;37:S31–2.
25. Timmers MS, Graafland N, Bernards AT, Nelissen RG, van Dissel JT, Jukema GN. Negative pressure wound treatment with polyvinyl alcohol foam and polyhexanide antiseptic solution instillation in posttraumatic osteomyelitis. Wound Repair Regen. 2009; 17:278–86. [PubMed]
26. Bernstein BH, Tam H. Combination of subatmospheric pressure dressing and gravity feed antibiotic instillation in the treatment of postsurgical diabetic foot wounds: a case series. Wounds. 2005; 17:37–48.
27. Kim PJ, Attinger CE, Steinberg JS, Evans KK. Negative pressure wound thera- py with instillation: past, present, and future. Surg Technol Int. 2015; 26:51–56.
28. Kim PJ, Attinger CE, Crist BD, et al. Negative pressure wound therapy with instilla- tion: review of evidence and recommendations. Podiatr Today. 2015;suppl:1-20.
29. Gabriel A, Kahn K, Karmy-Jones R. Use of negative pressure wound therapy with automated, volumetric instillation for the treatment of ex- tremity and trunk wounds: clinical outcomes and potential cost-effectiveness. Eplasty. 2014; 14:e41.
30. Kim PJ, Attinger CE, Steinberg JS, et al. The impact of negative-pressure wound therapy with instillation compared with standard negative-pressure wound therapy: a retrospective, historical, cohort, controlled study. Plast Reconstr Surg. 2014; 133(3): 709–716.
31.Gupta S, Gabriel A, Lantis J, Téot L. Clinical recommendations and practical guide for negative pressure wound therapy with instillation. Int Wound J, 2015. [PubMed]
32.Clinician panel recommendations for use of negative pressure wound therapy with instillation. McKanna M, Geraci J, Hall K, Hauan B, Howell M, Huey T, Lucius A, Mendez-Eastman S, Purcell K, Raizman R, Shepherd D, Gabriel A. http://www.o-wm.com/files/owm/Acelty_Supp_0416.pdf Ostomy Wound Manage. 2016;April: 1–14.