
Dr. Vita Boyar, an Assistant Professor of Pediatrics at Zucker School of Medicine, Hofstra University, is a Board-Certified Neonatologist and a Certified Wound Specialist Physician, practicing both neonatology and wound care at Cohen Children’s Medical Center of NY, Northwell Health System. Dr. Boyar is the only wound -certified neonatologist in the US and developed Northwell Neonatal Wound Service, the only one of its kind in the area. Dr. Boyar is the author of monthly pediatric column for Wound Management and Prevention Journal, “Children with wounds, asking the right questions”. Dr. Boyar is a member of Society for Pediatric Research. Her research interests include antimicrobial products in neonatal population, skin maturation, point-of-care ultrasound as a technique to assess skin injuries as well as novel, safe methods to prevent and treat pediatric cutaneous injuries. Her quality improvement projects have focused on pressure injury prevention. Dr. Boyar leads a Pressure Injury Prevention group at Cohen Children’s, part of nationwide Children’s Hospitals for Safety Initiative, and also serves as Co-Chair of the Northwell Pressure Ulcer Task Force. Dr. Boyar has published and is a reviewer for various journals including Wounds, Journal of Perinatology, Journal of Dermatology, Journal of Wound Ostomy and Continence Nursing, Pediatric Dermatology, International Wound Journal and Dermatologic Therapy. Dr. Boyar is a member of the Association for the American Academy of Pediatrics, Association for the Advancement of Wound Care, American Professional Wound Care Association and serves as a board member of International Society of Pediatric Wound Care. She has traveled both nationally and internationally to teach about pediatric wound care and has served as a consultant to various international NICUs in their skin team initiatives.
Boyar_Current Dialogues in Wound Management_2020_Article_6
Incontinence-associated dermatitis (IAD) or diaper dermatitis (DD) is the most common cutaneous condition,affecting young infants and neonates.1 DD can present as erythema, edema, erosions, weepy exudate or bleeding areas, involving the buttocks, perineum, groin and anal areas. It is also possible that skin folds may be involved or spared. The cause of DD is multifactorial and certain conditions can predispose or exacerbate the degree of dermatitis. Most cases of DD are of the irritant variety, with some being due to allergic factors or secondary to infectious etiology, including viral, bacterial and fungal. Prevention of DD is the gold standard with the goal of treatment being to allow restoration (despite ongoing exposure) and reduction in irritant penetration. This can be accomplished by providing a robust yet easily removable barrier-elements of which can contribute to epithelial healing.
Pathogenesis
The skin’s ‘barrier’ function is regulated by the outermost layer of the epidermis known as the stratum corneum (SC). The immaturity of the neonatal SC may predispose infants to dermatitis development. The corneocytes within the SC are held together by corneodesmosomes or “rivets”. A complex mixture of free fatty acids, phospholipids, glucosylceramides, cholesterol and ceramides produced by epidermal lamellar bodies fill in the crevices between the cells.2 The epidermal lamellar bodies also deliver critical antimicrobial proteins and the ceramides in the SC barrier are highly saturated, hydrophobic and essential for the formation of a permeability barrier. In preterm babies, the amount of fatty acids, ceramides and overall antimicrobial defenses are diminished, leading to increased stratum corneum permeability and susceptibility to inflammation.3 Additionally, preterm neonates lack vernix, a major contributor of the building blocks of the SC leading to further susceptibility to skin permeability and inflammation.
Multiple factors associated with diapers, bowel movements and overall skin condition can disrupt the development of physiologic homeostasis. Urination (babies may urinate over 10-12 times/ day) leads to exposure to ammonia and alkaline pH with persistent moisture keeping pH levels elevated and leaving the skin prone to maceration.4 Stool enzymes (lipase and protease) are also active around alkaline pH, which leads to excessive outer lipid breakdown and may contribute to a defective SC. The acidic pH level of the skin provides an “acid mantle” which is necessary for physiologic maturation, protection from pathogenic microbes and optimal enzyme function. The acidic pH level of the skin depends on the presence of lactic acid, free amino acids and fatty acids on the surface of the SC.5 Skin acidification is essential for the epidermal barrier maturation and repair process; especially for the activity enzymes responsible for SC lipid processing, that undergo increased degradation under basic pH, which can lead to enhanced desquamation.6 This physiologic pH disadvantage contributes to greater incidences of DD in the first few weeks in preterm and term neonates. Furthermore, microscopic skin breakdowns may allow chemical and microorganism entry, leading to inflammation, edema, erythema, further skin injury and eventually denuded outer epidermis.2, 3
Certain illnesses can predispose neonates and infants to DD such as viral gastroenteritis which produces liquid, frequent stool or bacterial sepsis which may contain inflammatory mediators and bacteria. Malabsorption syndromes can increase bacterial and carbohydrate load, along with higher stool pH, contributing to increased irritation. Short gut syndromes shorten the intestinal transit time, producing undigested food, bacterial overgrowth and caustic enzymes exposure. Fecal enzymes, bile acids and irritants are known to degrade SC proteins, causing breaks in skin, leading to irritants entry, inflammation, erythema, edema and potentially denuded, bleeding surfaces. Those with congenital metabolic syndromes often require enzyme supplements that can be caustic to the compromised skin. Neonates born with neonatal abstinence syndrome (exposure to illegal or prescription drugs in-utero) often have diarrhea and severe diaper rash.
Wearing occlusive diapers protects the outside but changes the inside microclimate, increasing moisture, humidity and even temperature leading to more alkalotic skin pH, propensity for maceration and prolong exposure to elements.7
Treatment Options
Traditional barrier products
Various preparations, such as creams, pastes and ointments are available DD treatment options on the market.8 Most contain an occlusive, such as petrolatum or dimethicone, as a hydrophobic layer on the skin providing exogenous barrier to water loss. The most common product in this category is petrolatum, a thicker occlusive, that may hasten evaporation (if applied in an overly thick layer). This can be detrimental in very preterm babies, where water vapor accumulation under the stratum corneum will cause microscopic breaks in the outer layer. A thin layer of application may alleviate this concern. Dimethicone is the second most common occlusive and is of a thinner consistency, with better water-vapor permeability.9 It is less occlusive in nature and is from a silicone family and is oil-free. Both can act as emollients, moisturizers that improve skin texture by filling in the crevices between corneocytes, adding softness.
Natural oils and herbal extracts are often added to diaper creams to provide essential fatty acids, but one has to be particularly aware of “natural oils” as an additive as many are known for their irritant potential, in addition to their lack of regulation by appropriate governing bodies. Herbal extracts may contain alcohol and cause significant allergies in pediatric patients.
Zinc oxide is the second most common ingredient with fluid repellant, anti-inflammatory, mild astringent and antiseptic properties, all of which have been shown to stabilize injured cells and halt inflammation.4 Zinc is an essential trace element and a co-factor for many cellular enzymes and plays a critical role in nucleic acid metabolism. Wound healing studies have shown that zinc may promote epithelial healing by enhancement of cell division and repair, through its structural integrity of dermal and mucosal tissue (by serving as a cofactor in fibroblast production of collagen and proteins) and via its role in an immune function as a mild antiseptic.10
Most products contain some amount of preservative to increase shelf life and slow down bacterial growth. Consumers are fearful of many additives, such as parabens due to their potential estrogenic effect, although studies are inconclusive in this regard. Preservatives are needed because of the risk of contamination between application of diaper creams, double dipping and tube touching.
Most practitioners would agree that deciding what skin protectant to use is confusing. No standard protocols exist describing the safest, most efficacious and cost-effective combination of products. Despite known benefits, classic tube-based skin protectants evoke concerns (Table 1).

TOUCHLESS CARE™ Zinc Oxide Protectant Spray
TOUCHLESS CARE™ Zinc Oxide Protectant Spray (Crawford Woundcare Ltd, Knutsford, UK) aims to bypass a few of the mentioned concerns. It is a multipurpose skin care treatment with 25% zinc oxide and 20% dimethicone with a spray application technique. The high dimethicone percentage facilitates the skin’s lubrication while ensuring easy clean-up and breathability. The anti-fungal version (TOUCHLESS CARE™ Antifungal Spray) is available as well, containing 2% miconazole nitrate, 10% zinc oxide and 10% dimethicone. Finally, TOUCHLESS CARE™ Clear Protectant Spray is a non-zinc formulation, containing 25% petrolatum and 20% dimethicone.
TOUCHLESS CARE™ Zinc Oxide Protectant Spray Use in Neonates
We evaluated TOUCHLESS CARE™ Zinc Oxide Protectant Spray with a cohort of 40 term and preterm neonates at a free-standing children’s hospital in a level 4 neonatal intensive care unit (NICU) and sister hospital level 3 NICU. The main objective was to gain an understanding of clinical usability of this product in a neonatal population and compare preliminary efficacy results in diaper dermatitis prevention and treatment to current products used in neonatal units. The secondary objective was to evaluate nursing and parents’ acceptability and ease of application/removal.
TOUCHLESS CARE™ Zinc Oxide Protectant Spray was evaluated against a unit-based guideline on IAD that our neonatal wound service had developed. It was a severity-based approach, incorporating 3 tube-based products, starting with prevention formulation (zinc oxide 12%, dimethicone, petrolatum mixture), progressing to moderate diaper dermatitis ointment containing additional repellant products (karaya gum) and higher zinc concentration (20%) along with Vitamin E, to a hydrophilic paste (with zinc, petrolatum, dimethicone and karaya) or crusting for denuded/bleeding areas in addition to cyanoacrylate liquid skin protectant in cases of significant maceration. Fungal DD requires a slightly different preparation, incorporating anti-fungal products. Two common antifungal preparations contain either nystatin or azoles (such as miconazole). The line of products we use has a convenient way of staying with the same product, except the anti-fungal version (incorporating miconazole).
TOUCHLESS CARE™ Spray Application
TOUCHLESS CARE™ Spray application was tested on neonatal mannequins to determine initial directions for application by members of the neonatal wound service team. Final application instructions include:
• Shake the bottle well for 5 seconds.
• Prime the sprayer away from the patient into a trash can or gauze by pumping until consistent spray is achieved.
• For neonates >28 weeks: dispense 2 pumps (1 to each buttock) from 2-3 inch distance.
– Smaller neonates may have sufficient covering with just 1 pump, 2-3 inches away to the midline.
• Do NOT allow product to run or over apply.
• Keep the diaper open for 1-2 minutes to allow slight drying to avoid significant diaper transfer.
Diaper changes were performed according to the unit’s protocol, every 3 hours along with vital signs.
Results
Study participants varied according to the following characteristics: very premature to full-term neonates with cardiac disease, kidney failure, liver failure, intestinal anomalies, neonatal abstinence syndrome, severe encephalopathy, and patients with mild intolerance or normal frequent elimination patterns. The average gestational age was 31 (27-38) weeks, with a minimum 10-day evaluation. Thirty-three babies had healthy skin and 7 had mild dermatitis at trial commencement (Table 2).

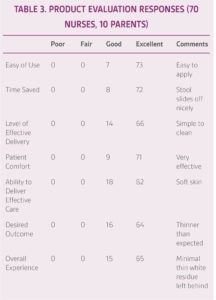
Evaluation forms were collected from 70 nurses and 10 parents over a 1-month period regarding use of TOUCHLESS CARE™ Spray. The data collected included ease of use, time saved, effective delivery to the skin, patient comfort, ability to deliver effective patient care, desired outcomes, and overall experience. Scores and subjective comments were also recorded including whether nurses preferred their current products vs. TOUCHLESS CARE™ Spray and whether they felt cross contamination was reduced.
Following application, a thin coating of product was retained on the skin at the 3-hour mark (Figure 1). Three out of 33 neonates developed mild dermatitis, a rate similar to prior incidence rates. Mild dermatitis improved in 5/7 neonates that started out with mild dermatitis (Figure 2), while 2 neonates progressed to the next step of the treatment protocol (Figure 3).
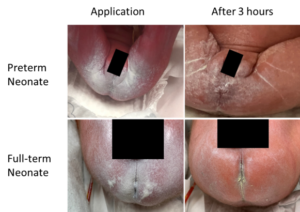
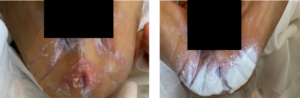
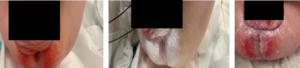
Caregivers reported ease of use and that the skin felt soft and lubricated (Table 3). Removal of stool was reported as easy to clean requiring 1-2 wipes, comparted to 2-4 wipes with tube creams, facilitating faster care. TOUCHLESS CARE™ Spray was deemed to be protective with 80% of respondents stating that it was equivalent to current therapy. Respondents appreciated the thinner consistency and touchless aspect of the product (90%), with 70% of respondents stating they would replace their current prevention product (Table 3).
We questioned whether use of an aerosolized product in the enclosed isolette would have an effect on the respiratory status of a preterm infant. From our observations, no side effects or respiratory decompensation were observed while using TOUCHLESS CARE™ Spray on patients housed in isolettes. The product spray was contained and narrow, without splattering, and did not travel past the diaper area by the subjective observation. No product inhalation was noted.
Discussion
It is difficult to demonstrate a superiority of one diaper dermatitis product over another, as their formulations are somewhat similar. A few published trials have demonstrated the superior efficacy of petrolatum and zinc compared to petrolatum alone.8 I personally favor formulations with a few key ingredients such as zinc, dimethicone and small amount of white petrolatum, with or without karaya or cellulose gum, and no additional additives. Since the active ingredients in these products are largely similar across the many available formulations, mode of delivery and surface retention may be important differentiators when selecting a product for use.
Limited data exists regarding TOUCHLESS CARE™ Spray use. In a study by Milne, TOUCHLESS CARE™ Spray was compared with a traditional tube ointment. After seven days of clinical use, the spray showed decreased bacterial growth in product cultures.11 The author concluded that a touchless product may reduce bacteria reintroduction to patients. Decreasing cross-contamination of everyday products and surfaces is of paramount importance for the hospitalized patient, especially the vulnerable neonatal population. It is reasonable to expect that neonatal cross-contamination would be similarly decreased with a touchless spray product as has been observed with adult data.
A poster presented by Roberts and colleagues demonstrated easier application and removal of the touchless product compared to traditional tube application.12 Marxen et al compared glove and product waste following touchless use and two ointments and was able to demonstrate a reduction in cost due to lower utilization of consumables.13 Similarly, Tolentino et al reported a lower cost of utilization with a touchless product compared to the current standard of care in a hypothetical care model.14 Taken together, these small studies indicate potential clinical and economic benefits of using TOUCHLESS CARE™ Spray for IAD treatment.
We are pleased to share the results of our study which include superior ease of application and removal, and satisfaction with efficacy and safe care delivery. While this was only an initial evaluation, we are in the process of executing a larger trial which will include endpoints associated with efficacy and pharmacoeconomic parameters.
In conclusion, it is feasible to apply TOUCHLESS CARE™ Zinc Oxide Protectant Spray in preterm and full-term neonates, with preliminary data showing similar efficacy in prevention and treatment of mild diaper dermatitis, and subjectively superior application satisfaction by caregivers as compared to thicker, tube-derived barrier creams/ointments.
Patient data and photos courtesy of Dr. Vita Boyar.
As with any case study, the results and outcomes should not be interpreted as a guarantee or warranty of similar results. Individual results may vary depending on the patient’s circumstances and condition.
NOTE: Specific indications, contraindications, warnings, precautions and safety information may exist for these products. Please consult a healthcare provider and product instructions for use prior to application. Rx only.
References
- Bender JK, Faergemann J, Skold M. Skin Health Connected to the Use of Absorbent Hygiene Products: A Review. Dermatology and Therapy 2017;7(3):319-330. doi:10.1007/s13555-017-0189-y.
- Menon GK, Cleary GW, Lane ME. The structure and function of the stratum corneum. Int J Pharm 2012;435(1):3-9. doi:10.1016/j.ijpharm.2012.06.005.
- Visscher MO, Adam R, Brink S, Odio M. Newborn infant skin: physiology, development, and care. Clin Dermatol 2015;33(3):271-280. doi:10.1016/j.clindermatol.2014.12.003.
- Esser M. Diaper Dermatitis: What Do We Do Next? Advances in Neonatal Care 2016;16(Suppl 5S):S21-S25. doi:10.1097/ANC.0000000000000316.
- Ali SM, Yosipovitch G. Skin pH: from basic science to basic skin care. Acta Derm Venereol 2013;93(3):261-267. doi:10.2340/00015555-1531.
- Fluhr JW, Darlenski R, Taieb A et al. Functional skin adaptation in infancy – almost complete but not fully competent. Exp Dermatol 2010;19(6):483-492. doi:10.1111/j.1600-0625.2009.01023.x.
- Visscher M, Odio M, Taylor T et al. Skin care in the NICU patient: effects of wipes versus cloth and water on stratum corneum integrity. Neonatology 2009;96(4):226-234. doi:10.1159/000215593.
- Visscher MO. Update on the use of topical agents in neonates. Newborn and Infant Nursing Reviews 2009;9(1):31-47.
- Nolan K, Marmur E. Moisturizers: reality and the skin benefits. Dermatol Ther 2012;25(3):229-233. doi:10.1111/j.1529-8019.2012.01504.x.
- Cario E, Jung S, Harder D’Heureuse J et al. Effects of exogenous zinc supplementation on intestinal epithelial repair in vitro. Eur J Clin Invest 2000;30(5):419-428. doi:10.1046/j.1365-2362.2000.00618.x.
- Milne CT. Hands-off! Using a spray application delivery system to impact bacterial contamination of moisture barriers [abstract]. Presented at the 48th WOCN Society Conference, June 4-8, 2016, Montreal, Canada 2016.
- Roberts S, Warde D, Marxen A, Thompson J, Stephenson C. A comparison of the application, removal and cost effectiveness of zinc oxide barrier products. Presented at Wild on Wounds 2015.
- Marxen A, Jackson S, Stephenson C. An assessment of the cost benefits of zinc oxide spray vs. tube application [abstract]. Presented at the 49th WOCN Society Conference, May 19-23, 2017, Salt Lake City, UT 2017.
- Tolentino AC, Dick S. Economic Model for Cost Containment: Evaluating the Utilization of a Touch-free Zinc Oxide in an Acute Care Hospital. Cureus 2019;11(6):e4957. doi:10.7759/cureus.4957.
©Copyright 2020 3M. All rights reserved. 3M and the other marks shown are marks and/or registered marks. Unauthorized use prohibited. PRA-PM-US-02394 (04/20).

