
Alisha Oropallo, MD, FACS, FSVS, FABWMS is a Professor of Surgery at the Donald and Barbara Zucker School of Medicine at Hofstra University/Northwell and the Feinstein Institutes for Medical Research. She practices as the Director of the Comprehensive Wound Healing Center at Northwell Health and Program Director of the Wound and Burn Fellowship program at Northwell Health. Dr. Oropallo completed her residency in Vascular Surgery at Boston University Medical Center and her General Surgery residency at Maimonides Medical Center, NY. She has been the lead investigator on multiple clinical trials, published papers, and has been invited to present lectures in wound healing globally. She is interested in education, research, and quality measures in tissue regeneration to help improve patient outcomes. Dr. Oropallo is a paid consultant for 3M.
Oropallo_Current-Dialogues-in-Wound-Management_2023_Article-2
Pressure injuries are frequent in older adults in whom the most common site reported is the sacral region. These injuries are caused by tissue shear, moisture, friction, and muscle atrophy leading to pathologic bone prominence.1 Recent clinical studies have demonstrated that the dermal matrix becomes thinner, increasingly crosslinked, and fragmented with advanced age. These structural changes along with cell senescence result in altered collagen fiber remodeling and increased stiffness.2 The altered environment of aged skin may also have a significant effect on age-related delays in healing.2 Pressure injuries can lead to ulcerations and, often, cannot be adequately assessed due to slough or necrotic tissue at the wound base. However, many of these unstageable ulcerations undergo sharp excisional debridement in an operating room, where anesthesia may be required leading to inherent risks, especially in patients with multiple comorbidities. One option for management of the pressure injury includes cleansing of the wound bed which can be accomplished with the use of 3M™ Veraflo™ Therapy. The design of Veraflo Therapy allows for cyclic delivery and dwell of a topical solution followed by removal of the topical wound solution, infectious materials, debris, and thick exudate. Veraflo Therapy has shown promise in chronic, complex wounds and has potential to help manage pressure injuries of multiple anatomical locations, such as sacral, ischial, greater trochanter, shoulder, etc. A recent meta-analysis of 13 clinical studies comprising 720 patients concluded that significantly fewer surgical debridements were performed (p = 0.01) and reduced time to closure (p = 0.03) in patients receiving Veraflo Therapy compared to patients receiving standard of care dressings.3 Additional case series reviews on the use of Veraflo Therapy in sacral or ischial pressure injuries have demonstrated similar findings. Veraflo Therapy has been shown to help irrigate the wound, remove fibrinous debris, and promote granulation tissue formation, and was associated with fewer operative debridements and decreased length of hospital stay compared to traditional negative pressure wound therapy.4 Similarly, a previously published case report noted effective wound cleansing and the development of granulation tissue with use of Veraflo Therapy in the management of a patient with sacral osteomyelitis and sepsis with pressure ulcer infection in combination with sharp debridement and systemic antibiotic therapy.1 Previously, Veraflo Therapy had two different dressings available for use (3M™ V.A.C. Veraflo™ Dressing and 3M™ V.A.C. Veraflo Cleanse™ Dressing). The properties of these initial dressings allowed for even distribution of topical wound solutions across the wound bed.5 In 2015, the 3M™ V.A.C. Veraflo Cleanse Choice™ Dressing was developed with three pieces, two pieces for varying wound depths and one with through holes to help facilitate the removal of thick exudate. More recently, 3M™ Veraflo Cleanse Choice Complete™ Dressing has become available in the US and Canada. This dressing iteration is a combination of a thin cover layer and a wound contact layer with through holes, providing clinicians two dressing options in one product. In April 2023, 3M™ Veraflo™ Therapy with either 3M™ Veraflo™ Cleanse Choice Complete™ Dressing or 3M™ V.A.C. Veraflo Cleanse Choice™ Dressing received the first clearance for hydromechanical removal of infectious materials, non-viable tissue and wound debris, which reduces the number of surgical debridements required, while promoting granulation tissue formation, creating an environment that promotes wound healing. This indication is only available in the US at this time. A case report detailing my experience with Veraflo Therapy utilizing Veraflo Cleanse Choice Complete for management of a sacral decubitus ulcer is presented below.
CASE REPORT
A 51‑year‑old woman was hospitalized with a history of multiple comorbidities including: Type 2 diabetes mellitus, hypertension, hyperlipidemia, end-stage renal disease on hemodialysis, electrolyte abnormalities, seizures, ventilator dependent respiratory failure with tracheostomy, persistent fevers, and an episode of cardiac arrest. The patient was found to have aspiration pneumonia with positive blood cultures for Streptococcus viridans and anginosus with carbapenem-resistant Enterobacteriaceae (CRE) in sputum, complicated with an unstageable sacral decubitus ulceration. The wound measurements for the sacral ulcer were 5.9 cm x 5.4 cm with a square surface area of 15.3 cm2 and was 75% covered with slough (Figure 1).
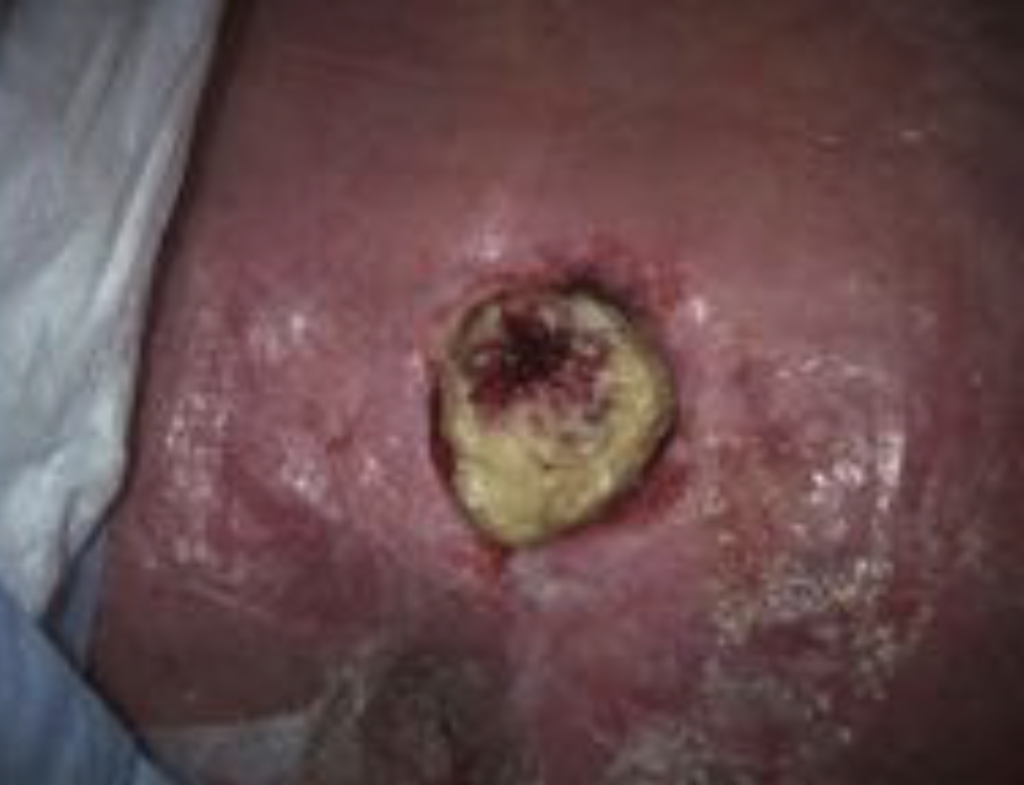
Image courtesy of Alisha Oropallo, MD, FACS, FSVS, FABWMS.
Due to the patient’s multiple comorbidities, Veraflo Therapy with Veraflo Cleanse Choice Complete Dressing was applied for 48 hours to help cleanse the wound bed and to solubilize and remove thick exudate and slough. Veraflo Therapy parameters included instillation of normal saline with a dwell time of 10 minutes, followed by continuous negative pressure at -125 mmHg for 3.5 hours.
Within 48 hours Veraflo Therapy with Veraflo Cleanse Choice Complete Dressing helped disrupt, soften, solubilize, and remove thick exudate and slough (Figure 2). Slough can not only impair wound healing but can be a nidus for infection. The wound measurement post-treatment was 5.6 cm x 2.9 cm. A 46.5% reduction in slough was noted along with a 56.5% increase in granulation tissue in the wound bed as estimated with diagnostic imaging. The wound bed demonstrated a significant increase in granulation tissue with decreased fibrinous exudate. Wound biopsy revealed vancomycin-resistant enterococcus (VRE) infection. Ongoing antibiotics including linezolid were administered. Percutaneous endoscopic gastrostomy was placed for nutritional support. Due to the improvement of wound, the patient was eligible for discharge to a post-acute care setting where the transition to 3M™ V.A.C.® Therapy could be continued.
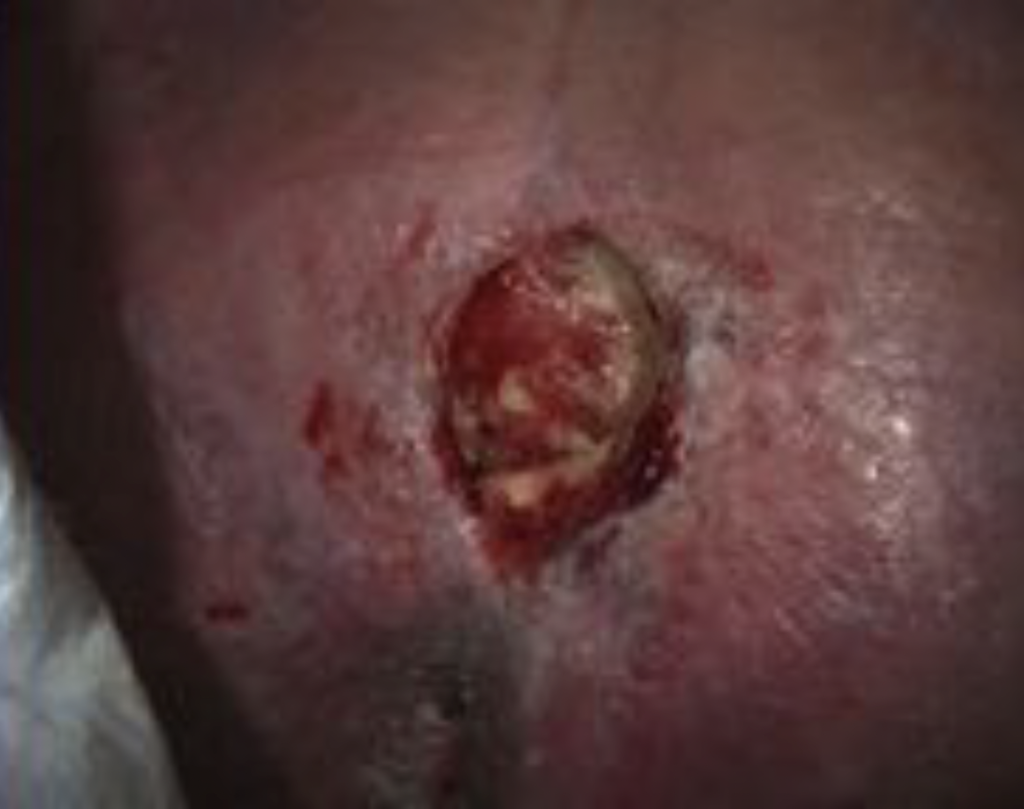
Image courtesy of Alisha Oropallo, MD, FACS, FSVS, FABWMS.
In my facility, convention is that wounds should demonstrate more than 60% fibrinous exudate or slough to demonstrate the best results with Veraflo Therapy. Dwell time and topical wound solutions used vary depending on the wound size, amount of exudate, and personal preference regarding the topical wound solution. Recent international consensus guidelines recommended the use of saline as an instillation solution of choice.6 However, as various topical antiseptic solutions become more widely available, there may be opportunities to improve outcomes further with topical antiseptic instillation therapy to help assist in adjunctively managing infection along with use of systematic antibiotics. The consensus guideline update also noted that in addition to good clinical practice, such as use of systemic antibiotics, topical antiseptic solutions may be utilized in wounds with acute infection or high levels of bacterial colonization, though use should be limited to 24 to 48 hours to avoid potential cytotoxic effects.6 Similarly, a recent case series has demonstrated the efficacy of the use of a dilute hypochlorous acid solution with Veraflo Therapy in various wound types.7 Granulation tissue development was observed in all wounds and wound closure was achieved in a majority of patients (9/10) with the use of Veraflo Therapy and the hypochlorous acid solution for instillation.7
Veraflo Therapy plays a role in promoting wound healing whether it necessitates flap reconstruction, skin grafting or patient discharge home with a stable chronic wound, with continuation of wound care.4 The majority of clinicians have embraced Veraflo Therapy in promoting wound healing. Yet, optimal parameters for specific wounds including interface material, and the amount and duration of negative pressure application, are needed. Understanding the mechanism of
biofilm formation and the use of concomitant cellular tissue-based products with Veraflo Therapy to help accelerate healing requires additional study.
Depending on the therapy objectives, Veraflo Therapy can be used to cleanse the wound, promote granulation tissue or a combination of both.11 In this case, this patient was not a candidate for flap reconstruction or grafting, thus, therapy goals were wound cleansing and removal of infectious materials and thick exudate. In this patient, use of Veraflo Therapy with Veraflo Cleanse Choice Complete Dressing allowed for complete wound cleansing and biopsy to direct the course of treatment. Additionally, this device fostered the transition to V.A.C.® Therapy to continue the patient’s wound care. The use of Veraflo Therapy with Veraflo Cleanse Choice Complete Dressing should be considered for patients with complex, hard-to-heal wounds, wounds with debris or thick exudate, and when surgical debridement must be delayed.
References
- Sugita M, Higami S, Sawamoto T, Morita S, Nakagawa Y. Severe sacral region pressure ulcer infection treated in negative pressure wound therapy with instillation and dwelling: a case report. Tokai J Exp Clin Med. 2022;47(2):52-55.
- Blair MJ, Jones JD, Woessner AE, Quinn KP. Skin Structure-Function Relationships and the Wound Healing Response to Intrinsic Aging. Adv Wound Care. 2020;9(3):127-143. doi:10.1089/wound.2019.1021
- Gabriel A, Camardo M, O’Rorke E, Gold R, Kim PJ. Effects of Negative-Pressure Wound Therapy With Instillation versus Standard of Care in Multiple Wound Types: Systematic Literature Review and Meta-Analysis. Plast Reconstr Surg. 2021;147(1S-1):68S-76S. doi:10.1097/PRS.0000000000007614
- Arowojolu OA, Wirth GA. Sacral and Ischial Pressure Ulcer Management With Negative-Pressure Wound Therapy With Instillation and Dwell. Plast Reconstr Surg. 2021;147(1S-1):61S-67S. doi:10.1097/PRS.0000000000007613
- Lessing C, Slack P, Hong KZ, Kilpadi D, McNulty A. Negative pressure wound therapy with controlled saline instillation (NPWTi): dressing properties and granulation response in vivo. Wounds. 2011;23(10):309-319.
- Kim PJ, Attinger CE, Constantine T, et al. Negative pressure wound therapy with instillation: International consensus guidelines update. Int Wound J. 2020;17(1):174-186.
- Delapena S, Fernandez LG, Foster KN, Matthews MR. Negative Pressure Wound Therapy With Instillation and Dwell Time for the Management of Complex Wounds: A Case Series. Wounds. 2020;32(12):E96-e100.
- Fernandez L. The utility of negative pressure wound therapy with instillation for wound bed preparation. Chronic Wound Care Manage Res. 2019;6(2019):51-58. doi:10.2147/CWCMR.S198418
- Miller JM, Binnicker MJ, Campbell S, et al. A Guide to Utilization of the Microbiology Laboratory for Diagnosis of Infectious Diseases: 2018 Update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin Infect Dis. 2018;67(6):813-816. doi:10.1093/cid/ciy584
- Sartelli M, Guirao X, Hardcastle TC, et al. 2018 WSES/SIS-E consensus conference: recommendations for the management of skin and soft-tissue infections. World J Emerg Surg. 2018;13:58. doi:10.1186/s13017-018-0219-9
- Gupta S, Gabriel A, Lantis J, Teot L. Clinical recommendations and practical guide for negative pressure wound therapy with instillation. Int Wound J. 2016;13(2):159-174.
NOTE: Specific indications, contraindications, warnings, precautions, and safety information exist for these products and therapies. Please consult a clinician and product Instructions for Use prior to application. Rx only.
As with any case study, the results and outcomes should not be interpreted as a guarantee or warranty of similar results. Individual results may vary depending on the patient’s circumstances and condition.
Patient data and images courtesy of Alisha Oropallo, MD, FACS, FSVS, FABWMS.
© 2023 3M. All rights reserved. 3M and the other marks shown are marks and/or registered marks. Unauthorized use prohibited. Used under license in Canada.

