Karen has worked in the medical device industry for over 25 years in the areas of product management and medical information/publications for several companies, including Philips Respironics (Westminster, CO), OSNovative Systems (Santa Clara, CA) and Acelity (San Antonio, TX). In her current position as a medical writer, Karen assists health care professionals worldwide in preparing manuscripts regarding wound care outcomes and techniques for publication in various medical journals. Karen earned a Bachelor of Science degree from University of Colorado

Julie M. Robertson received a Bachelor of Science in Molecular and Cellular Biology from Texas A&M University and a PhD in Immunology from the University of Texas Graduate School of Biomedical Sciences in Houston. Following graduation, she became a post-doctoral fellow and a scientific writer for the laboratory of Dr. Judith James at the Oklahoma Medical Research Foundation. During her time in Oklahoma, Dr. Robertson gained valuable scientific and medical writing experience in the field of autoimmune disease through manuscript, abstract, poster, and grant writing. After leaving Oklahoma, Dr. Robertson became a medical writer at Acelity where she has helped health care professionals publish wound care manuscripts in peer reviewed journals.

Ricardo Martinez has worked in the Medical Device Industry for over 25 years in the areas of Product Development, Regulatory Affairs and Medical Information and Publications for distinguished companies such as Adeza Biomedical, Sunnyvale CA; SA Scientific and Acelity of San Antonio, TX. As the Sr Director of Medical Information and Publications for Acelity, Mr. Martinez leads an innovative team of Medical Information and Publication specialists that support a network of health care professionals (HCPs) from around the world. In his current work in Publications, Mr. Martinez assists HCPs to publish their medical findings and techniques in areas of wound care and regenerative medicine in various journals.Mr. Martinez holds a Master’s of Science degree from Texas A&I University, Kingsville, TX.
Beach, Robertson and Martinez_Current Dialogues in Wound Management_2019_Volume 5_Issue 1
INTRODUCTION
An increased prevalence of chronic wound treatment in the outpatient setting has shifted costs from hospital inpatient to outpatient settings.1 According to a recent study of US Medicare beneficiaries, the highest annual Medicare expenditures (2014) for wound care categorized by site of service were for hospital outpatients ($9.9–$35.8 billion), followed by hospital inpatients ($5.0–$24.3 billion).1These high expenditures have prompted increased scrutiny and pressure on providers to justify use of all wound care products and therapies within new value-based models for reimbursement.
Meanwhile, new generations of sophisticated dressing interfaces and devices, including mechanically powered negative pressure wound therapy (NPWT), are being commercialized to satisfy specific needs of patients with wounds. A single-use, mechanically powered disposable NPWT (dNPWT) has been introduced for patients with smaller, lower-exudating wounds who may benefit from negative pressure and removal of exudate and tissue debris.3;4 This ultraportable technology is powered by specialized springs, does not require batteries or electrical power, and addresses many of the constraints associated with traditional NPWT use (such as device weight and noise level) for active, mobile patients.5;6 However, few studies address best practices to optimize its value in clinical practice.
A panel meeting of physicians and nurses with experience using dNPWT was convened to develop recommendations surrounding the use of dNPWT as delivered by the SNAP™ Therapy System. This publication summarizes the original published manuscript of panel member recommendations7 to help guide appropriate use of the SNAP™ Therapy System.
SNAP™ THERAPY SYSTEM
The SNAP™ Therapy System consists of a cartridge with activation/reset key and a dressing with integrated tubing (Figure 1). The cartridge is set to deliver a preset level of negative pressure. Priming the cartridge with the activation/reset key initiates delivery of negative pressure. Inside the cartridge, exudate is turned into a gel for containment. The dressing is made of an advanced hydrocolloid sealing layer and a blue reticulated open-cell foam interface. The hydrocolloid layer is meant to help provide periwound protection and easier removal, while assisting with maintaining a seal and reducing maceration. A ring-shaped hydrocolloid dressing accessory (SNAP™ SecurRing™ Hydrocolloid) is available and recommended by panel members to help seal the dressing, particularly in difficult contoured areas.7 Dressings should be changed at a minimum of twice weekly, with the frequency adjusted by the clinician asappropriate.
Panel members stressed that a major benefit of the SNAP™ Therapy System is that it may allow a quick return to normal activities of daily living.7 The device is silent, thus patients can return to work and social activities without being self-conscious about therapy. The dressing and cartridge are small enough to be hidden out of sight (patient’s leg, arm, or belt) and not interfere with mobility. Tubing should be cut to fit and positioned in a way that does not cause the patient to trip.
SNAP™ THERAPY SYSTEM
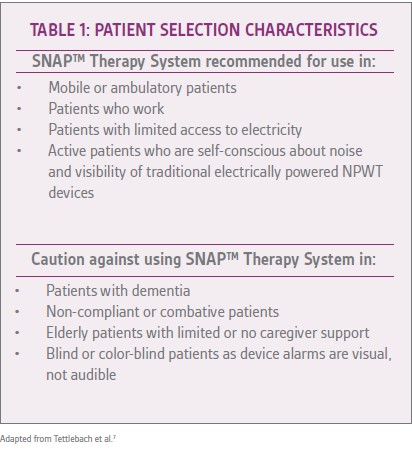
Panel members emphasized the importance of proper patient selection for successful use of the SNAP™ Therapy System (Table 1).7Prior to application of the SNAP™ Therapy System, the panel members recommended addressing all patient and wound-centered factors influencing the wound’s ability to heal (including infection, bioburden/biofilm, and elevated metalloproteinases, if suspected).
RECOMMENDED WOUND TYPES FOR SNAP™ THERAPY SYSTEM USE
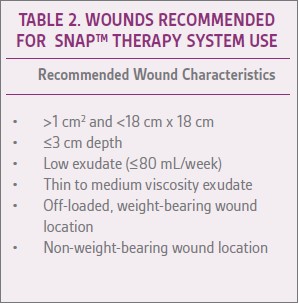
Selecting appropriate wound types for treatment with the SNAP™ System is important for optimal outcomes. In general, the SNAP™ Therapy System is best suited for small- to medium-sized wounds with low exudate (Table 2).7Use of the SNAP™ Therapy System is not recommended in cases of active wound infection (e.g., redness, swelling, pain, purulent exudate).
Infection should be successfully treated prior to initiating the SNAP™ Therapy System. Panel members recommended that clinicians, caregivers, and patients frequently monitor the patient’s wound, surrounding tissue, and secreted exudate for signs of infection or other secondary conditions such as maceration, dermatitis, or wound bed tissue deterioration during SNAP™ Therapy System use.
BEST PRACTICES IN APPLYING THE SNAP™ THERAPY SYSTEM
Just as with standard V.A.C.® Therapy, achieving a long-lasting dressing seal around the wound can be challenging. Panel members recommended developing a uniform technique of applying the SNAP™ Therapy System dressing for best results.7 Most panelists recommended using skin preparation and the ring-shaped accessory SNAP™ SecurRing™ Hydrocolloid for a high percentage of wounds to better obtain a seal.7(Table 3)lists several dressing application techniques to optimize outcomes.
USE OF SNAP™ THERAPY SYSTEM WITH OFFLOADING OR COMPRESSION
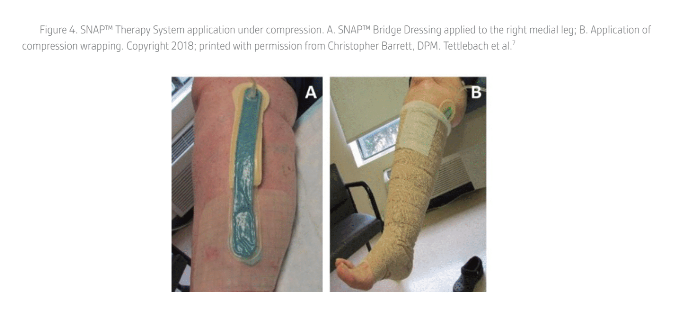
The SNAP™ Therapy System can be used with offloading or compression. However, the panel members recommended that the SNAP™ Dressing be placed safely and efficiently under a total contact cast (TCC) and that a “bridging” technique may be helpful during offloading or compression as the long foam bridge can help position the dressing port and tubing away from the wound and minimize pressure points (Figures 2-4)
SNAP™ THERAPY SYSTEM REIMBURSEMENT
The panel members also discussed reimbursement of the SNAP™ Therapy System using information specific to US guidelines, and to clarify requirements for obtaining reimbursement from Medicare and commercial payors.
Mechanically-powered NPWT systems are classified as nondurable single patient use medical equipment with separate reimbursement codes for the device. Once the SNAP™ Therapy System is placed on a qualified patient, the outpatient provider (wound care center or home health agency) bills Medicare or the commercial insurance company for reimbursement.
Effective January 1, 2015, Healthcare Common Procedure Coding System codes G0456 and G0457 were deleted and replaced by new Current Procedural Terminology codes: 97607 and 97608. These 2 codes apply only when an entirely new, complete therapy device (cartridge/pump/canister) and dressing are provided with wound assessment, application of therapy, and patient education. A list of codes and descriptions can be found in further detail in the Tettlebach et al article.
CONCLUSIONS
Understanding when and how best to use advanced wound care therapies, including the SNAP™ Therapy System, is paramount to clinical success and cost containment. Systematic methods of training home health providers and other clinicians to identify patient and wound characteristics that would benefit from the SNAP™ Therapy System as well as to properly apply the dressing, including obtaining a seal, are necessary to provide high-quality care.
References
1.Nussbaum SR, Carter MJ, Fife CE et al. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value in Health 2018;21(1):27-32. doi:10.1016/j.jval.2017.07.007.
2.Landers S, Madigan E, Leff B et al. The Future of Home Health Care: A Strategic Framework for Optimizing Value. Home Health Care Management and Practice 2016;28(4):262-278. doi:10.1177/1084822316666368.
3.Fong KD, Hu D, Eichstadt S et al. The SNaP system: biomechanical and animal model testing of a novel ultraportable negative-pressure wound therapy system. Plast Reconstr Surg 2010;125(5):1362-1371.
4.Fong KD, Hu D, Eichstadt SL et al. Initial clinical experience using a novel ultraportable negative pressure wound therapy device. Wounds 2010;22(9):230-236.
5.Armstrong DG, Marston WA, Reyzelman AM, Kirsner RS. Comparative effectiveness of mechanically and electrically powered negative pressure wound therapy devices: A multicenter randomized controlled trial. Wound Repair Regen 2012;20(3):332-341.
6.Bradbury S, Walkley N, Ivins N, Harding K. Clinical Evaluation of a Novel Topical Negative Pressure Device in Promoting Healing in Chronic Wounds. Adv Wound Care 2015;4(6):346-35
7. doi:10.1089/wound.2014.0596.7.Tettlebach W, Arnold J, Aviles A et al. Use of mechanically powered disposable negative pressure wound therapy: recommendations and reimbursement update. Wounds 2019;31(2 Suppl):S1-S17.