
Born and raised in Syracuse, NY, Austin Cavelli earned her bachelor’s degree in psychobiology from Binghamton University. She has done extensive research investigating the neurobiology of alcohol addiction as an undergraduate student as well as the aging brain while working as a research assistant at Columbia University Medical Center. At the same time, she worked as a research coordinator and medical assistant in the vascular surgery department at Weill Cornell Medical College, providing the clinical experience necessary to attend PA school. In 2014, Austin graduated from Weill Cornell Graduate School of Medical Sciences with a master’s of science and a physician assistant’s certificate, specializing in surgery. She is board certified through the National Commission of Certification of Physician Assistants (NCCPA) and is licensed in the state of New York. Austin started her career as a physician assistant at the Hospital for Special Surgery in 2014, focusing on adult reconstruction and joint replacements. Her duties include first assisting in the operating room as well as seeing patients in clinic, including initial consults for both surgical and non-operative care management, as well as routine post-op care. She also works as a medical consultant for KCI as well as Flexion Therapeutics, allowing her the opportunity to collaborate on research projects and provide product-related education at medical conferences nationwide.
Cavelli_Current Dialogues in Wound Management_2020_Article 2
Introduction
Surgical site complications (SSC) are a leading cause of re-admission, surgical revision and overall patient dissatisfaction. Despite the surgical procedure going according to the preoperative plan, even a seemingly minor wound complication can lead to serious consequences. Furthermore, surgical site complications, particularly surgical site infections (SSIs) are extremely costly. Of the top 5 healthcare-acquired infections, surgical site infection represents 33.7% of the $9.8B cost to the US healthcare system each year.1
The number of revision joint arthroplasties performed in the United States continues to increase with time and SSIs; following aseptic and septic total joint arthroplasty (TJA), revisions are well documented.2 The published literature examining incision management across a number of different surgical procedures has reported positive clinical benefits in patients using PREVENA™ Incision Management System compared to standard of care dressings,3-6 specifically to reduce surgical site complications.
In 2016, use of the PREVENA™ System, was introduced in our practice. Its application and utilization was initially limited to complex revision cases; however, over time, our inclusion criteria expanded based on the beneficial outcomes and overall reduction in SSCs that were observed. The greater the number of risk factors for development of SSCs and severity, the more likely we would apply the PREVENA™ Therapy System postoperatively. Of course, clinical evaluation of patients is important, and the use of the PREVENA™ Therapy System is ultimately determined by the provider on a case-by-case basis.
Although revision arthroplasty has the same goal as primary TJA, revision surgery is often longer and more complex, requiring an extended or alternative approach to the surgical incision, and thus, it increases the risk of surgical site complications. I present the case of a complex revision total knee replacement which illustrates our best efforts to salvage a patient’s limb after he was told amputation was his only reasonable treatment option.
Case Study
A 64-year-old male with underlying comorbidities of rheumatoid arthritis, deep vein thrombosis and hypertension presented to our outpatient clinic with concern about a recurrent infection after right total knee replacement. His medication included a daily anti-hypertensive; he denied the use of steroids and antibiotics. Upon examination, his vital signs were stable, with symptoms limited to a draining sinus with localized erythema and edema over the right knee (Figure 1).
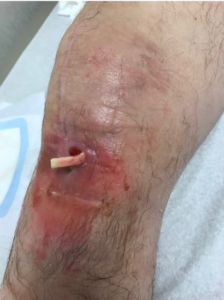
The patient was conducting daily wound debridement and packing with betadine-soaked gauze. He was told by his previous surgeon that above-the-knee amputation was his only treatment option.The patient had an extensive past surgical history. A right total knee arthroplasty (TKA) was performed in 2010. In 2014 he developed an acute infection of the right knee for which he underwent irrigation and debridement with exchange of the poly-ethylene plastic insert. Following this, he had a total of five revision knee surgeries for recurrent infection. The first revision surgery was performed in fall 2014 and at that time an antibiotic spacer was placed, with preservation of the metal femur and an all-poly tibial component. His symptoms improved temporarily until spring of 2015, at which point a patellar tendon augmentation with synovectomy was performed. In 2016, a femoral component antibiotic spacer was placed, and the all-poly tibial component was revised. In the summer of 2016, the femoral component and the patella were revised including placement of a metal femoral component. By fall, the metal femoral component was again revised for recurrent infection. Cultures consistently showed methicillin-sensitive staphylococcus aureus (MSSA) throughout his course of treatments. The patient confirmed treatment with a total of six previous courses of intravenous antibiotics via a peripherally inserted central catheter (PICC) line after each knee surgery. The patient’s lab results revealed elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels of 37mm/hour and 6.4mg/L respectively. The most recent aspiration confirmed cultures growing MSSA.
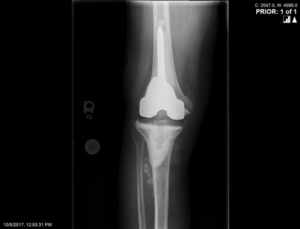
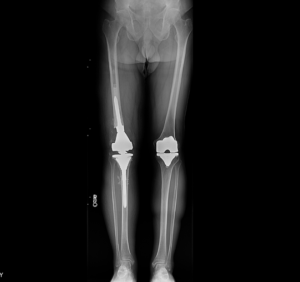
Radiographic images at his initial consultation with us revealed a revision, stemmed femoral prosthesis fully cemented, and a cemented all-poly tibial component in satisfactory alignment. The patellar button was loose with migration proximally and there was evidence of osteomyelitis at the anterior tibia. (Figure 2).
The metal, poly and cement were maintained to some degree in each revision. The complete removal of all these components at one time was never performed during any of his revision surgeries. For that reason, the decision was made to proceed with a two-stage revision TJA for complete removal. Consideration of an anterior flap was discussed with the patient at the time of the consultation due to the poor tissue coverage and compromised skin after multiple revision surgeries.
Stage one of the two-stage revision was performed in the fall of 2017. All existing hardware and cement were removed and an antibiotic spacer was placed using rush rods and antibiotic impregnated cement. Immediately following incision closure, a PREVENA PEEL & PLACE™ Incision Management System – 20 cm was applied under sterile conditions in the operating room. The dressing remained in place for 7 days postoperatively and was eventually removed by the visiting home nurse.
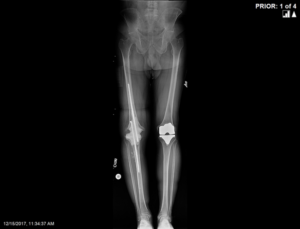
The patient was treated with 6 weeks of intravenous antibiotics and the antibiotics were discontinued for 3 weeks thereafter in preparation for re-aspiration of the right knee to ensure the infection was fully cleared. The re-aspiration was negative and the second surgery, reimplantation of hardware, was scheduled approximately 3 months after the initial operation. A distal femoral replacement was chosen based on the degree of bone loss in this patient (Figure 3). The skin was tenuous; however, full coverage was obtained and a flap was not deemed necessary at the time of closure. Again, a PREVENA PEEL & PLACE™ Incision Management System – 20 cm was applied immediately post-operatively under sterile conditions in the operating room.
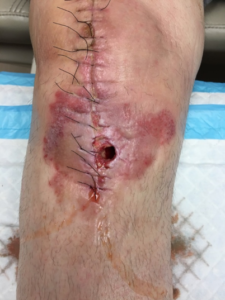
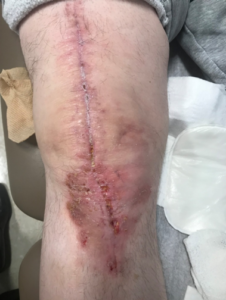
Ten days postoperatively, the patient informed us that the wound started to drain during physical therapy with flexion exercises. The patient was brought into the office immediately for evaluation and documentation of the wound (Figure 4). The 2 x 2 cm opening at the distal aspect of the incision over the anterior tibia was observed. There was a moderate amount of serosanguinous drainage without purulence, the patient did not complain of any fever, chills, malaise and his vital signs were stable.
Given the patient’s extensive history of recurrent infection as well as the placement of a distal femoral replacement, the safest course of action was to bring the patient back to the operating room for a full irrigation and debridement of the knee, exchange of the polyethylene plastic liner and re-closure of the wound. The procedure was performed without complications. A PREVENA PEEL & PLACE™ Incision Management System – 20 cm was used postoperatively and remained in place for 7 days. The patient’s range of motion exercises were delayed initially to limit tension and allow proper healing of the wound. The patient was seen in the office at postoperative day 21 and again at postoperative day 90 (Figures 5 and 6, respectively).
The wound remained clean, dry, and intact. The patient did not report any increases in pain, and made excellent progress with physical therapy, despite the initial restrictions to allow the wound to heal. Radiographic images were obtained and confirmed all components were in good position without evidence of complication (Figure 7). In summary, the patient remains symptom free, without recurrent infection and the above-the-knee amputation was avoided, resulting in a patient who could not be happier with his outcome.
Discussion
The PREVENA™ System has become an integral part of our practice for both primary and revision total joint arthroplasty patients. Despite the complexity of this case, this patient is one of many examples where the PREVENA™ System was extremely useful. To further assess the use of the PREVENA™ System in minimizing surgical site complications, we have retrospectively investigated 90-day complication rates, particularly following complex revision total joint arthroplasty in our practice. There has been a notable reduction in wound complication rates as compared to what has been documented in the literature. Our use of the PREVENA™ System has undoubtedly evolved over time; however, what has remained consistent is our clinical focus on preventing surgical site complications and providing the highest quality of care to our patients and the PREVENA™ System has definitely contributed to our success.
As with any case study, the results and outcomes should not be interpreted as a guarantee or warranty of similar results. Individual results may vary depending on the patient’s circumstances and condition.
NOTE: Specific indications, contraindications warnings, precautions and safety information exist for PREVENA™ Therapy. Please consult the applicable PREVENA™ System Clinician Guide instructions for use prior to application. Rx only.
References
1. Zimlichman E, Henderson D, Tamir O et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Internal Medicine 2013;173(22):2039-2046.
2. Rasouli MR, Restrepo C, Maltenfort MG, Purtill JJ, Parvizi J. Risk factors for surgical site infection following total joint arthroplasty. J Bone Joint Surg Am Vol. 2014;96(18):e158. doi:10.2106/JBJS.M.01363.
3. Cantero R, Rubio-Perez I, Leon M et al. Negative-Pressure Therapy to Reduce the Risk of Wound Infection Following Diverting Loop Ileostomy Reversal: An Initial Study. Adv Skin Wound Care 2016;29(3):114-118. doi:10.1097/01.ASW.0000480458.60005.34.
4. Pachowsky M, Gusinde J, Klein A et al. Negative pressure wound therapy to prevent seromas and treat surgical incisions after total hip arthroplasty. Int Orthop 2012;36(4):719-722.
5. Kwon J, Staley C, McCullough M et al. A randomized clinical trial evaluating negative pressure therapy to decrease vascular groin incision complications. J Vasc Surg 2018;68(6):1744-1752. doi:10.1016/j.jvs.2018.05.224.
6. Grauhan O, Navasardyan A, Hofmann M, Muller P, Stein J, Hetzer R. Prevention of poststernotomy wound infections in obese patients by negative pressure wound therapy. J Thorac Cardiovasc Surg 2013;145(5):1387-1392.
@Copyright 2020 3M. All rights reserved. 3M and the other marks shown are marks and/or registered marks. Unauthorized use prohibited. PRA-PM-US-02322 (03/20).

