
Dr. Bauer Sumpio received his medical degree in 1980 and his Ph.D. in Physiology in 1981 from Cornell University Medical College in New York. He was involved in post-graduate training in General Surgery at Yale University and underwent fellowship training in Vascular Surgery at the University of North Carolina. Dr. Sumpio is currently a tenured Professor of Surgery (Vascular), Radiology (Interventional) and Medicine (Cardiology) at Yale University School of Medicine, the Emeritus Chief of Vascular Surgery at the Yale-New Haven Hospital and Emeritus Program Director of Vascular Surgery Fellowship Program. Dr. Sumpio is a consultant for 3M.
Sumpio_Current-Dialogues-in-Wound-Management_2021_Article_2
Venous leg ulcers are the most frequently occurring chronic lower extremity wounds with a prevalence of 0.9%.1 Treatment costs are estimated to be $1 billion (US) with a corresponding loss of about a million workdays per year.1,2
Lower extremity venous hypertension is the major underlying factor in developing venous ulceration.3,4 The elevated venous pressures reduce the pressure gradient for perfusion and the resulting venous stasis hinders the clearance of catabolic products. Venous hypertension causes transudation of serous fluid and red blood cells into the subcutaneous tissue. Hemoglobin from the red blood cells breaks down to produce the pigment hemosiderin, leading to hyperpigmentation, especially in the medial paramalleolar areas. The primary cause of venous hypertension is insufficiency of the valves of the deep venous system and the perforating veins of the lower leg.
DIAGNOSIS
Patients with ulcers due to venous insufficiency usually complain of aching and swelling of the legs. They may recount a history of recurrent cellulitis, previous deep vein thrombosis, or previous superficial venous surgery. Symptoms are often worse at the end of the day, exacerbated when the leg is dependent, and relieved by leg elevation. In limbs of patients with venous insufficiency, there is evidence of chronic edema and stasis dermatitis. This eczematic process may spread from the area of the medial malleolus and involve the leg circumferentially. The recurrent cellulitis can cause contraction of the subcutaneous tissue in the lower third of the leg, below the knee, and together with the chronic edema can produce a “bottle leg” appearance.
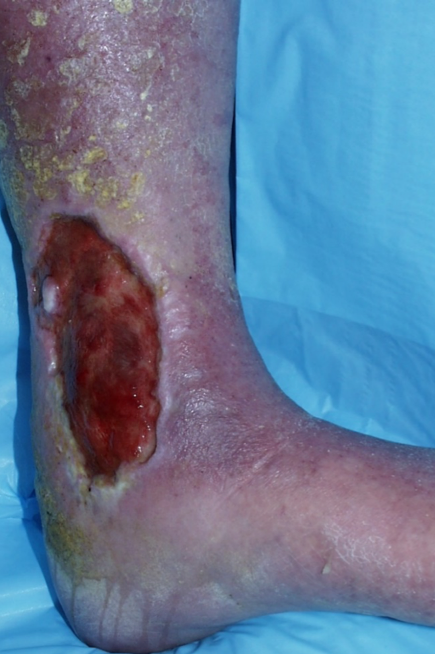
Venous ulcers rarely present in the foot and are commonly located in the “gaiter” distribution of the leg, around the medial malleolus, where the venous pressures are highest (Figure 1). The ulcers are surrounded by areas with induration and brown pigmentation of the surrounding area (brawny induration) and scaling skin. These ulcers are often exquisitely tender and weep copious serous fluid.
ASSESSMENT
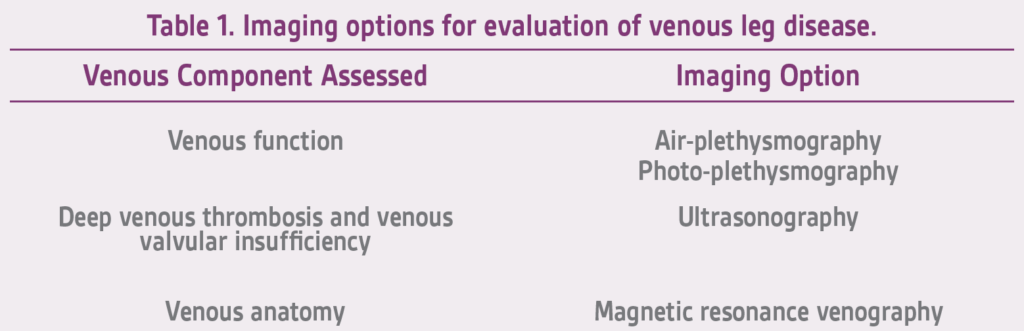
Diagnostic evaluation of venous legdisease encompasses the use of the non-invasive vascular laboratory in conjunction with diagnostic imaging (Table 1) such as ultrasound, and rarely, magnetic resonance venography (MRV).5
Both air and photo-plethysmography can also be used to assess the venous vasculature. A photoelectrode placed on the medial calf measures the rate of venous capillary refill following emptying after exercise (after five calf compressions normal refill times should be less than twenty seconds). The test is repeated with an occlusive tourniquet impeding flow in the superficial veins but not the deep veins. Normalization of the refill time indicates superficial venous incompetence whereas a continued abnormal refill time confirms deep venous insufficiency. Air plethysmography can be used to quantify limb segment volume changes and the time course of these changes in response to various maneuvers including leg elevation, dependency and exercise.
The diagnosis of venous disorders including deep venous thrombosis and venous valvular function are usually assessed with ultrasonography. Normal veins exhibit phasic flow changes with respiration, no retrograde flow with the Valsalva maneuver or proximal limb compression and augmentation of flow with distal limb compression. In addition, normal veins will collapse with the application of light pressure from the doppler probe. Duplex ultrasonography is currently the test of choice for diagnosing deep venous thrombosis in the superficial femoral, popliteal, and calf veins. Thrombus may or may not be visualized; however, flow disturbances in and of themselves may provide the diagnosis.
Flow abnormalities which suggest thrombosis include: nonphasic or absent flow with continuous flow proximal to the occlusion and minimal or no augmentation, incomplete or absent vein compressibility, and increased flow may be seen in collateral superficial veins. Inferior vena cava and iliac vein flow abnormalities can be identified; however, compression cannot be performed at these sites. Systematic limb compression and Valsalva maneuvers can help identify areas of valvular incompetence, in which case reversal of flow is seen.
MRV has rapidly come to the forefront as a non-invasive venous imaging modality and serves as a supplemental method to assess the venous anatomy of patients with severe IV contrast allergy, contrast induced nephropathy or slow venous flow. MRV is the most sensitive and specific test for the assessment of deep and superficial venous disease in the lower legs and pelvis, areas not accessible by means of other modalities. MRV is particularly useful because it can help detect previously unsuspected nonvascular causes of leg pain and edema when the clinical presentation erroneously suggests venous insufficiency or venous obstruction.
While multiple non-invasive and invasive methods are available to assess the venous circulation, it should be obvious that not every patient requires an exhaustive battery of tests in order to evaluate their venous status. In general, only those tests which are likely to provide information that will alter the course of treatment and management should be performed.
MANAGEMENT
It is clear that the management of lower extremity ulcers caused by venous insufficiency must include measures that improve the abnormal venous blood return from the affected extremity. Edema control, surgical correction of selected underlying pathology, and local wound care are all important components of the treatment plan.6
Edema Control
Elevation of the leg is a simple maneuver that can effectively but temporarily eliminate venous hypertension. All patients should be encouraged to elevate the affected leg above the level of the heart for 2 to 3 hours during the day and when lying in bed at night. Compression therapy is also effective in controlling edema and accelerates healing of ulcerations. However, before compression is applied to the limb, significant occlusive arterial disease should be excluded. Compression therapy is generally contraindicated in patients with an ankle-brachial index (ABI) less than 0.5, arterial occlusive disease, and heart failure. Many different types of compression devices are available, including elastic and non-elastic bandages, compression therapy systems (3M™ Coban™ 2 Two-Layer Compression System and 3M™ Coban™ 2 Lite Two-Layer Compression System), graduated compression stockings, and compression pumps. The most effective way of delivering compression must be determined on an individual basis. Compression should be applied just before arising from bed and removed at bedtime.
Surgical Correction
The goal of surgical treatment of venous insufficiency is to correct the underlying pathology.6 Open surgical or endovascular intervention can result in healing up to 90% of ulcers and provide modest long-term results if the diagnostic studies can adequately characterize and localize the incompetent superficial or perforating system valves. Ulcer recurrence is significantly diminished after superficial venous surgery or ablation and use of compression stockings when compared with compression therapy alone.6 If reflux exists in the deep venous system, ligation and stripping or ablation of the superficial veins has a poor result and high ulcer recurrence rate. For patients who are young and understand the importance of long-term compression therapy and adjunctive antiplatelet or anticoagulant therapy, reconstruction of vein valves can be recommended.
It should be emphasized that acute or chronic arterial vascular insufficiency may be superimposed on the changes of chronic venous insufficiency, impairing the healing of the venous ulcer. In these situations, lower extremity revascularization may be required to assist in healing a venous ulceration that is not responding appropriately to compression therapy. Furthermore, the presence of significant lower extremity swelling or skin changes can complicate arterial reconstructions by altering the surgical approach to distal arterial target sites.
Local Wound Care
The goal of local wound care in patients with venous ulcers is to minimize stasis; decrease bacterial contamination of the ulcer; and provide a healthy, moist wound environment that promotes healing. Table 2 provides a brief list of available local wound care options. The healthcare provider should select local wound care based on needs of the wound, patient comfort, ease of use, and cost.7

SKIN PROTECTION
Treatment of stasis dermatitis minimizes further trauma to the skin from scratching. Pruritus can be controlled by topically applied corticosteroids or orally administered antihistamines, or both. Barrier films (such as 3M™ Cavilon™ No Sting Barrier Film and 3M™ Cavilon™ Advanced Skin Protectant) may also help reduce periwound skin breakdown caused by an overly moist wound environment.
INFECTION MANAGEMENT
In the presence of infection and cellulitis, oral antimicrobial therapy should be instituted based on the suspected pathogen and clinical findings. Severe heavily contaminated venous ulcers should be treated with broad-spectrum intravenous antibiotics with particular emphasis on the role of biofilms. In cases of gross wound infections and rampant cellulitis, use of a silver-containing medication, such as silver sulfadiazine 1%, may be useful in the initial setting to reduce the bacterial load. The predominant organisms cultured from chronic ulcers are gram-positive pathogens like Staphylococcus aureus and Streptococcus pyogenes. The most common gram-negative bacteria are Pseudomonas aeruginosa, especially in the diabetic population. The addition of silver dressings may help manage the venous ulcer infection when used along with antibiotics.
Aggressive mechanical debridement is an important cornerstone for effective wound care. Sharp debridement in the operating room or at the bedside, when applicable, allows for thorough removal of all necrotic material and optimizes the wound environment. All necrotic material should be excised, and the degree of penetration of the infection should be established. Foot soaks, whirlpool therapy, or enzymatic debridement have a use, but are rarely effective and may lead to further skin maceration or wound breakdown.
OPTIMIZE WOUND ENVIRONMENT
Various moisture-retentive dressings can be used in conjunction with compression therapy to relieve pain, debride necrotic tissue, and promote granulation tissue formation. An “ideal” dressing not only provides protection against further bacterial contamination but also maintains moisture balance, optimizes the wound pH, absorbs fibrinous fluids, and reduces local pain. Additionally, the use of moist dressings in clean, granulating wounds is recommended to enhance the wound environment. No prospective randomized studies have demonstrated the superiority of dressing products compared with standard saline wet-to- dry sterile gauze in establishing a granulation bed.8 Various dressings are currently available to target specific characteristics of the wound; however, moist normal-saline dressings are probably sufficient for most wounds. Wound healing should be monitored and type of dressing used switched when stalled or worsening wounds are observed.
Evidence for use of advanced wound therapy in venous ulcer management is limited. There are reports on the use of negative pressure wound therapy (NPWT) with and without instillation in the management of venous leg ulcers.9,10 These studies suggest that NPWT may be beneficial, though larger, randomized controlled trials are necessary. Recently, a large prospective randomized trial has been established to evaluate the efficacy of V.A.C.® Therapy in the management of venous ulcers (ClinicalTrials.gov Identifier: NCT03688841). However, this trial is ongoing and results have not yet been released.
The use of bioactive drugs (e.g., recombinant platelet derived growth factor, becaplermin) or skin substitutes (e.g., living cell skin substitute, human fibroblast-derived dermal substitute) have shown promising anecdotal results. However, as a recent Cochrane review suggests, only pentoxifylline in association with compression therapy has a sufficient level of evidence regarding the benefit in increasing the healing rate of chronic venous ulcers.11
In conclusion, venous leg ulcers are common and frequently challenging clinical presentations. A multi-disciplinary approach is needed to attain healing of these ulcers and reduce the risk of recurrence.
References
1. Nussbaum SR, Carter MJ, Fife CE, et al. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value in Health. 2018;21(1):27-32.
2. Phillips CJ, Humphreys I, Thayer D, et al. Cost of managing patients with venous leg ulcers. Int Wound J. 2020;17(4):1074-1082.
3. Sumpio BE. Foot ulcers. New England J Med. 2000;343(11):787-793.
4. Sumpio BE, Blume P, Mena-Hurtado C. Lower Extremity Ulceration. In: Creager M, Beckman J, Loscalzo J, eds. Vascular Medicine: A Companion to Braunwald’s Heart Disease. 3rd ed. Philadelphia, PA: Elsevier; 2020:836-848.
5. Collins KA, Sumpio BE. Vascular assessment. Clin Podiatr Med Surg. 2000;17(2):171-191.
6. O’Donnell TF, Jr., Passman MA, Marston WA, et al. Management of venous leg ulcers: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2014;60(2 Suppl):3S-59S.
7. O’Donnell TF, Jr., Lau J. A systematic review of randomized controlled trials of wound dressings for chronic venous ulcer. J Vasc Surg. 2006;44(5):1118-1125.
8. Sood A, Granick MS, Tomaselli NL. Wound dressings and comparative effectiveness data. Adv Wound Care. 2014;3(8):511-529.
9. Scarpa C, de Antoni E, Vindigni V, Bassetto F. Efficacy of negative pressure wound therapy with instillation and dwell time for the treatment of a complex chronic venous leg ulcer. Wounds. 2020;32(12):372-374.
10. Vuerstaek JD, Vainas T, Wuite J, Nelemans P, Neumann MH, Veraart JC. State-of-the-art treatment of chronic leg ulcers: A randomized controlled trial comparing vacuum-assisted closure (V.A.C.) with modern wound dressings. J Vasc Surg. 2006;44(5):1029-1038.
11. Jull AB, Arroll B, Parag V, Waters J. Pentoxifylline for treating venous leg ulcers. Cochrane Database Syst Rev. 2012;12:CD001733.
Patient photo courtesy of Bauer E. Sumpio, MD, PhD.
NOTE: Specific indications, contraindications, warnings, precautions and safety information exist for these products and therapies, some of which may be Rx only. Please consult a clinician and product instructions for use prior to application.
© 2021 3M. All rights reserved. 3M and the other marks shown are marks and/or registered marks. Unauthorized use prohibited. 3M marks used under license in Canda. All other marks are property of their respective owners.PRA-PM-US-02958 (02/21).

