
Dr. Robert Klein completed podiatric medical school in Chicago at the Rosalind Franklin University of Medicine and Science, Scholl College of Podiatric Medicine. Dr. Klein continued his surgical training as the Chief Resident at Michigan Health Center in Detroit. Dr. Klein is a Clinical Assistant Professor in the Department of Surgery at the University of South Carolina School of Medicine (USCSOM) Greenville and specializes in wound care, limb preservation, and surgery.
Klein_Current Dialogues in Wound Management_2019_Volume 5_Issue 1
INTRODUCTION
Diabetic wounds that extend to bone with abscess and/or bone infection may often require surgery, intravenous (IV) antibiotics, and advanced wound care modalities as part of the treatment plan. Surgery, including bone resection and possible partial amputation, is sometimes necessary for limb salvage. In addition to surgery, the use of negative pressure wound therapy (NPWT) can help promote granulation tissue and draw wound edges together. Utilizing complimentary sequential advanced wound care, including the use of PROMOGRAN PRISMA™ Matrix and epidermal grafting, can aid in the promotion of granulation tissue and wound healing, leading to wound closure.1-4
CASE STUDY
A type II diabetic male was referred to my practice with a chronic, non-healing diabetic ulceration. The patient stated that the ulceration started as a small blister and failed to respond to conventional wound care administered by his primary care provider. Conventional wound care included oral and topical antibiotics and off-loading in a surgical shoe. The ulceration increased in size and depth, and the patient developed cellulitis of the affected foot necessitating admission to the hospital for IV antibiotics, advanced wound care, and surgery. The patient’s past medical history was significant for poorly controlled diabetes, diabetic peripheral neuropathy, a history of tobacco use, alcoholism, hyperlipidemia, hypertension, and obesity.
On initial presentation, the patient had an extensive wound with cellulitis to the proximal ankle joint with lymphangitis and lymphadenopathy. Necrotic and devitalized tissue, unhealthy granulation tissue, and tunneling to the fifth metatarsal head and the proximal phalangeal base was noted. The fifth metatarsal phalangeal joint was exposed (Figure 1).
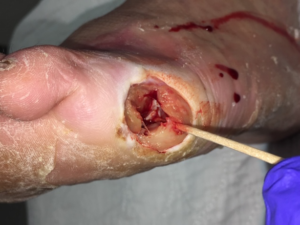 Figure 1. Wound at presentation
Figure 1. Wound at presentation
The patient was immediately admitted to the hospital where the work-up included routine admission laboratory tests, deep culture and sensitivity of the wound, plain film x-rays and a magnetic resonance imaging (MRI), chest x-ray, EKG, and an arterial Doppler with segmental pressures and ankle-brachial index (ABI) measurements. X-Ray and MRI results were suggestive for osteomyelitis of the base of the proximal phalanx of the fifth toe and distal one-third portion of the fifth metatarsal. The patient was counseled on his treatment options and opted for fifth toe amputation with partial fifth ray resection. Due to the extent of the wound and infection, a decision was made to keep the wound open post-operatively and utilize negative pressure wound therapy (NPWT) to promote granulation tissue development and promote wound healing.5
The patient was taken to the operating room (OR) on the second day of his admission for a fifth toe amputation as well as a partial fifth ray resection under local anesthesia with IV sedation (Figure 2).
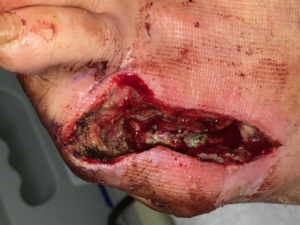 Figure 2. Patient’s foot after amputation
Figure 2. Patient’s foot after amputation
V.A.C.® Therapy at -125 mm Hg was started on post-operative day one. In order to protect the fragile structure, the exposed bone was covered with ADAPTIC TOUCH ™ Non-Adhering Silicone Dressing. The V.A.C.® GRANUFOAM™ Dressing was cut to fit the wound bed area (Figure 3).
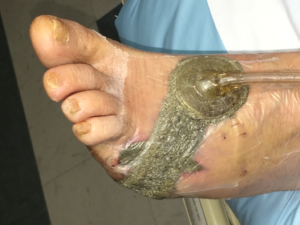 Figure 3. NPWT commenced on post-operative day one
Figure 3. NPWT commenced on post-operative day one
The dressing was placed into the wound bed and plantar ulceration and covered with an adhesive drape following the manufacturer’s directions. V.A.C.® Therapy was applied in such a way to cover the plantar ulceration with foam and bridge the NPWT to the larger surgical wound dorsally. V.A.C.® Therapy was utilized for 30 days. The patient remained non-weight-bearing with a knee rest walker to minimize pressure on the wound. Physical therapy was consulted for gait training and to prevent deconditioning.
At post-operative day 30, once the wound had significant healthy granulation tissue and the exposed bone and deeper structures were completely covered, PROMOGRAN PRISMA ™ Matrix, was used as an advanced wound care dressing to continue to support additional granulation tissue and to promote healing (Figure 4).
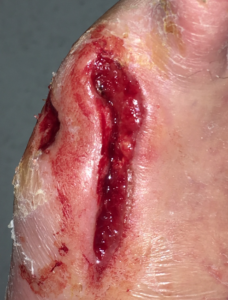 Figure 4.Wound care changed to PROMOGRAN PRISMA™ Matrix at post-operative day 30.
Figure 4.Wound care changed to PROMOGRAN PRISMA™ Matrix at post-operative day 30.
TIELLE ™ Hydropolymer Adhesive Dressing was applied over PROMOGRAN PRISMA™ Matrix to help maintain a moist wound environment. IV antibiotics were discontinued at this time, and the patient was placed on oral antibiotics for an additional two weeks under the direction of the infectious disease specialist.
On post-operative day 44, the plantar ulceration was completely closed and a decision was made to use epidermal grafting for the dorsal surgical foot wound. CELLUTOME™ Epidermal Harvesting System was used to isolate and harvest epidermal micrografts
The donor site was prepped with hair removal and a 70% isopropyl alcohol wash. The harvester was placed over the donor site, ensuring that complete contact was made with the skin. The vacuum head was attached to the harvester, warmth and negative pressure was applied to raise the epidermal micrografts over a period of 35 minutes. Once the epidermal micrografts had satisfactorily developed, the CELLUTOME™ System unit was powered off, followed by removal of the vacuum head. The micrografts were collected using ADAPTIC TOUCH™ Non-Adhering Silicone Dressing, and the micrografts were applied to the wound, followed by bolstering of the non-adherent contact dressing with wound closure strips to the area of the wound (Figure 5). The CELLUTOME™ System was removed, and the donor site was covered with a transparent film dressing. The wound site and donor site dressings were changed once a week according to the manufacturer’s instructions. The wound was completely closed 55 days post-op (Figure 6).
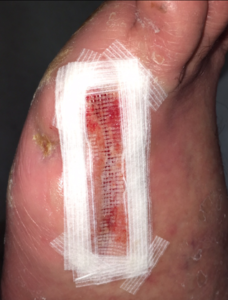 Figure 5. Micrografts applied to wound.
Figure 5. Micrografts applied to wound.
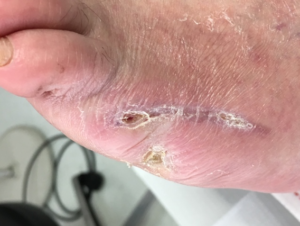 Figure 6. Wound closure at post-operative day 55.
Figure 6. Wound closure at post-operative day 55.
CONCLUSION
Diabetic ulcerations with bone infection can be a very challenging clinical scenario to treat. Many of these patients have complex co-morbid medical problems complicating or delaying healing. Diabetic patients with osteomyelitis are at increased risk for amputation and possibly loss of limb, as well as the morbidity and mortality associated with diabetic-related foot disease. Surgical treatment followed by the use of sequential advanced wound care contributed to the successful closure of this Wagner Grade 3 DFU.
References
1. Gupta S, Andersen C, Black J et al. Management of Chronic Wounds: Diagnosis, Preparation, Treatment, and Follow-up. Wounds 2017;29(9):S19-S36.
2.Frykberg RG, Banks J. Challenges in the Treatment of Chronic Wounds. Adv Wound Care 2015;4(9):560-582. doi:10.1089/wound.2015.0635.
3.Snyder RJ, Fife C, Moore Z. Components and Quality Measures of DIME (Devitalized Tissue, Infection/Inflammation, Moisture Balance, and Edge Preparation) in Wound Care. Adv Skin Wound Care 2016;29(5):205-215. doi:10.1097/01.ASW.0000482354.01988.b4.
4.Schultz GS, Sibbald RG, Falanga V et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen 2003;11(2 Suppl):S1-S28.
5.Langer V, Bhandari PS, Rajagopalan S, Mukherjee MK. Negative pressure wound therapy as an adjunct in healing of chronic wounds. Int Wound J 2015;12(4):436-442. doi:10.1111/iwj.12132.
Photos and patient information courtesy of Robert Klein, DPM, FACFAS, CWS, Collom & Carney Clinic Association, Texarkana, TX.
As with any case study, the results and outcomes should not be interpreted as a guarantee or warranty of similar results. Individual results may vary depending on the patient’s circumstances and condition.
NOTE: Specific indications, contraindications, warnings, precautions, and safety information may exist for Systagenix and KCI (Acelity companies) products. Please consult a healthcare provider and production instructions for us prior to application.

