
Kara Couch is a Family Nurse Practitioner and Certified Wound Specialist, who has been working in wound care for the past 13 years. She is a member of the Board of Directors for both the Association for the Advancement of Wound Care (AAWC) and the Alliance of Woundcare Stakeholders. Kara also serves as co-leader of the International Consolidated Venous Ulcer Guidelines for AAWC and has authored or co-authored numerous articles and chapters on wound healing. She lectures nationally on wound care and wound healing topics.
Couch_Current Dialogues in Wound Management_2016_Volume 2_Issue 2
DEFINITION OF WOUND INFECTION
In chronic wounds, prolonged inflammation leads to macrophage and neutrophil recruitment to the wound bed. There is then a release of pro-inflammatory cytokines and increased production of matrix metalloproteinases (MMPs) and a decrease of tissue inhibitors of metalloproteinases (TIMPs). This leads to degradation of the extracellular matrix and dysfunctional collagen deposition. This cellular cascade results in necrotic tissue, high bacterial burden and tissue breakdown.1Wounds are traditionally defined as being infected when bacteria reach 105 colonies per gram of tissue. This culture-based data, along with clinical signs of infection (calor, rubor, purulence, etc.), are combined to make the diagnosis of infection.
BACTERIA CONTAMINATION-INFECTION SPECTRUM
Open wounds are not sterile. There are always bacteria on the intact skin surface (i.e. coagulase negative staphylococcus aureus, yeast). This also populates on the surface of wounds. In healthy hosts, the immune system is able to overcome and progress a wound through the normal phases of healing. There is a spectrum of bacteria on wound beds that run from contaminated to colonized to critically colonized to infected (fig 1). Wounds that become critically colonized indicate increased bacterial colonies that are overwhelming the host. Signs of critical colonization include
•a non-healing wound (i.e. did not improve as expected over a period of time)
•increased edema
•exudate and debris in the wound bed.
If the bacterial overgrowth is not arrested in this stage, the wound may progress to be clinically infected and the patient may require systemic antimicrobial therapy. Sibbald et al described this process with an examination of the factors contributing to critical colonization and then infection.2,3 The concepts of Wound Bed Preparation and the DIME method of wound care reviews these principles in detail.
TYPICAL MANNER OF DIAGNOSING WOUND INFECTION
For decades, wound infection has been identified by tissue culture or biopsy, preferably quantitative cultures or biopsy. Tissue biopsies are invasive procedures that require training (not all wound clinicians are able to perform this procedure), are expensive and can possibly exacerbate an infection.4-6 However, studies have shown that tissue biopsies are accurate in detecting bacterial load with nearly 100% sensitivity, 90% specificity and 95% accuracy for predicting wound closure.4-6Many laboratories cannot process quantitative cultures. Therefore, clinicians rely on semi-quantitative swabs, which are much easier to obtain. The majority of wounds are treated on an outpatient basis. and the inability to capture accurate data can result in delays in healing and other wound healing complications. The swab process is not standardized, imprecise, time consuming (results take hours to days) and may not fully report polymicrobial bacteria on the wound bed. In addition, improperly collected swabs may lead to overtreatment with systemic antimicrobials which can lead to increased antibiotic resistance.
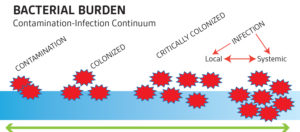 Figure 1. Source: www.worldwidewounds.com
Figure 1. Source: www.worldwidewounds.com
The manner in which semi-quantitative culture swabs are obtained is often variable. There are several validated techniques to obtain wound cultures. The Levine technique involves rotating the wound swab over a 1 cm2area of the wound for 5 seconds. The Z-swab technique involves rotating the swab in a zig-zag fashion across the wound without touching the wound edge. Both methods have been well studied, and evidence suggests that Levine is superior to the Z-swab technique. It is believed this is due to the Levine technique’s ability to express fluid from the wound bed and obtain samples from both the wound bed and slightly below the surface of the wound. The Levine swab may be useful for routine wound monitoring, but quantitative biopsies are preferred if there is suspicion of antibiotic-resistant organisms.
Key points of consideration are:
1.Routine cultures of clinically non infected wounds should be discouraged as they are of low yield.
2.Wounds must be cleaned of debris and exudate prior to culture. Swabbing the surface without cleansing has little utility.6
3.Swabbing pus is of low yield as well. It is important to know what lies beneath the wound surface in the subcutaneous tissue and on the wound bed.4-6
4.Swab viable and not necrotic tissue. Preferably, the wounds will be sharply debrided of any non-viable tissue prior to culture.
5.If the wound is dry, the culture swab should be moistened with saline as bacteria will attach better in this environment.6
6.Wound cultures taken in the home environment and in the absence of debridement are likely to be contaminated and, therefore. are relatively useless in determining a course of antimicrobial therapy.
7.Cultures serve as a guide for proper antimicrobial therapy, not an absolute indication of need.
DNA-BASED IDENTIFICATION METHODS
Analysis of DNA-based microbial identification methods demonstrates that the use of culture-based methods and subjective clinical signs/symptoms of infection grossly underestimate the complexity of the wound microbial burden.7-8 Polymerase chain reaction (PCR) and 16S rRNA sequencing are being used more commonly now to evaluate bacterial load. These tests are completed more rapidly than culture swabs. The 16S rRNA gene-based analyses found approximately 4 times more bacterial strains than the culture-based analyses. This discrepancy may lead to under treatment of a pathogen and over treatment of a colonizer. More studies are underway to further elucidate the most specific and sensitive methods to detect complex bacterial communities.7-8 This work will further the definitions of critical colonization vs. clinical infection and development of algorithms for treatment.
BIOFILM
Hurlow et al define biofilm as ‘‘a structured community of microbial cells enclosed in a self-produced polymeric matrix that is adherent to an inert or living surface.’’9 The presence of biofilm can indicate a stubborn group of microorganisms that become embedded into the wound and are generally intolerant of antibiotics, antiseptics and inflammation. Biofilm formation is known to exist in chronic wounds, particularly in those patients who also have implanted devices or foreign bodies such as suture material. Evidence shows they are also in acute wounds. Evaluations are ongoing to determine if biofilm precedes wound infection and how it affects overall healing. There is no validated screening or diagnostic tool available yet for biofilm detection.9 The impact of biofilm on culture-swab analyses and DNA-based bacterial analyses is unknown currently.
FUTURE TECHNOLOGY
Ideally, a wound clinician could swab a wound and have accurate results reported back while the patient was still being treated. Point-of-care (POC) testing for wound microbiomes, similar to glucometers checking blood glucose levels, is being evaluated currently. POC technology already exists (WOUNDCHEK™, WOUNDCHEK™ Laboratories, North Yorkshire, UK) and is use in Europe and Canada to detect an imbalance of proteases which lead to increased inflammation and bacteria proliferation.10 These results in turn aid in directing topical therapy appropriately to treat this imbalance with MMP stabilizing dressing, such as, oxidized reducing collagen (ORC). Other therapies which are in development include “smart” dressings which have microsensors to detect increasing levels of bacteria and which would either turn colors to alert patient and clinicians to heavy bioburden or which could send signals to a computer or smart device with the changes in microbial load.
Overall, wound clinicians have been reliant on culture-based analyses to determine infection despite its known limitations and flawed methodology for procurement. The scientific advances with POC technology, as well as DNA-based microbial detection, are changing how microbiome data is obtained and how that data can be utilized to treat wounds more effectively and to decrease the amount of antibiotic resistance in the wound patient population.
References
1.Mast BA, Schultz GS. (1996) Wound Repair Regen. 4:411-420
2. Sibbald RG, Woo KY, Ayello EA. Increased Bacterial Burden and Infection: The Story of NERDS and STONES. Adv Skin Wound Care. October 2006;19;447-61.
3.Sibbald RG, Orsted HL, Coutts PL, Keast DH. (July 2007). Best Practice Recommendations for Preparing the Wound Bed: Update 2006. Adv Skin Wound Care. 2007;20(7):390-405.
4. Copeland-Halperin LR, Kaminsky AJ, Bluefeld N, Miraliakbari R. Sample procurement for cultures for infected wounds: a systematic review. J Wound Care. 2016;25 Supp 4;S4-S10.
5. Angel DE, Lloyd P, Carville K, Santamaria N. The clinical efficacy of two semi-quantitative wound-swabbing techniques in identifying the causative organism(s) in infected cutaneous wounds. Int Wound J. 2011;8(2):176-85.
6. Bonham PA. Swab cultures for diagnosing wound infections: a literature review and clinical guideline. JWOCN. 2009;36(4);389-95.
7. Sprockett DD, Ammons CG, Tuttle MS. Use of 16SrRNA sequencing and quantitative PCR to correlate venous leg ulcer bacterial bioburden dynamics with wound expansion, antibiotic therapy and healing. Wound Repair Regen. Spe. 2015;23(5):765-71.
8. Price LB, Liu CM, Melendez JH, et al. Community analysis of chronic wound bacteria using 16S rRNA gene-based pyrosequencing: Impact on diabetes and antibiotic on chronic wound microbiota. PLoS One. 2009;4(7):e6462.
9. Hurlow J, Couch K, Laforet K, et al. Clinical Biofilms: A Challenging Frontier in Wound Care. J Adv Wound Care. 2015;4(5):295-301.
10.Snyder RJ, Driver V, Fife CE, et al. Using a Diagnostic Tool to Identify Elevated Protease Activity Levels in Chronic and Stalled Wounds: A Panel Discussion.

