Dr. Robert Snyder is Professor and Director of Clinical Research and Fellowship Director in wound care and research at Barry University SPM. He has created and implemented the first post residency Wound Management and Research Fellowship for podiatric medical schools. He is certified in foot and ankle surgery by the American Board of Podiatric Surgery and is also a board certified wound specialist. Dr Snyder is past-president of the Association for the Advancement of Wound Care and past-president of the American Board of Wound Management, the certifying body for Wound Care Specialists. In addition to his doctorate, he holds an MSc in Wound Healing and Tissue Science from Cardiff University. His expertise at Cardiff, Wales, was further acknowledged as he was selected as an Internal Marker for MSc dissertations with an Honorary Title. To constantly expand his knowledge and stay current in all aspects of healthcare, he is completing an MBA in Health Management. Dr. Snyder is a key opinion leader and sought after speaker, lecturing extensively throughout the United States and abroad. He was chosen to develop and teach a wound care course for physicians internationally. Dr. Snyder has published several book chapters and over 150 papers in peer reviewed and trade journals on wound care. He serves on the editorial advisory boards of Ostomy Wound Management, Wounds and Podiatry Management and has recently been appointed as a periodic reviewer for the Lancet and NEJM. He has been a Principal Investigator on more than 50 randomized controlled trials for innovative wound healing modalities and products.

Dr. Karim Ead is a recent graduate from Barry University School of Podiatric Medicine. Prior to attending medical school, he received his Master’s Degree in biomedical science with a concentration in Wound Healing & Tissue Regeneration. Dr. Ead has been involved in various research studies and has worked closely with wound care specialist Dr. Robert Snyder DPM, MSc, CWS. This summer he be will be starting his 3-year intensive foot & ankle surgical training at Westside Regional Medical Center with an emphasis on joint replacement, external fixation, arthroscopy, trauma, tibial shaft/plafond, ankle, hindfoot and forefoot reconstruction, podiatric research and wound management. His interests include podiatric medicine & surgery, diabetic & functional limb preservation and regenerative medicine among others.
Snyder and Ead_Current Dialogues in Wound Management_2019_Volume 5_Issue 1
INTRODUCTION
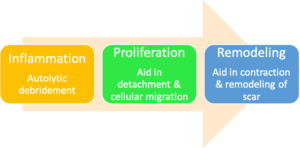
The goal of utilizing various wound care products throughout the treatment plan is to modulate the stages of wound healing which include inflammation, cell migration and proliferation, and tissue remodeling. Significant progress has been made over the years in understanding the science of wound healing and the development of various therapies and dressing options.
BIOCHEMISTRY OF CHRONIC WOUNDS
Wound healing represents a comprehensive series of well-orchestrated physiological reactions that create an orderly healing cascade. This process includes four key overlapping phases: hemostasis, inflammation, proliferation and remodeling.1 It progresses along a continuum that should result in the restoration of anatomical and function integrity. Acute wounds in a relatively healthy host will continue through the wound healing cascade quickly due to the robust utilization of intrinsic growth factors, cytokines and matrix proteins that keep the wound along a well-regulated trajectory.1 Various theories have been proposed to explain why some wounds become chronic and non-healing. Despite the etiological differences in chronic wounds, they often share common pathophysiologic features. First and foremost, the inflammatory phase of healing is prolonged due to increased pro-inflammatory biomarkers, high level of protease activity, and diminished growth factor and cellular activity.
Protease contribution came to surface when it was discovered that the extracellular matrix of chronic wounds did not present with intact fibronectin, a high molecular weight glycoprotein that is required for growth factor signaling and cell adhesion.2Matrix metalloproteases (MMP’s) represent a distinct class of 23 protein-degrading enzymes (proteases) and in normal physiologic instances they help modulate the wound healing trajectory (Figure 1). It has been found that MMP-8 and MMP-9 predominate in chronic wound environments.3 Destructive microbial communities secrete bacterial proteases that catalyze infectious processes via skin penetration and host immune evasion. This creates a more conducive environment for host and bacterial proteases to work synergistically to promote an infected wound bio-infectious network.4 The incursion of macrophages and neutrophils induce a cycle of non-progressive inflammation causing the inhibition of collagen formation and decrease in the production of key protease inhibitors. Advanced wound dressings with super absorbent properties may reduce protease activity by removing protease-containing wound fluid.
Growth factors were initially discovered for their ability to stimulate repeated homeostatic cycles mitosis of fibroblasts and epidermal cells.6 Growth factors may also be stored intra-cellularly (e.g., in platelet alpha granules) or by attaching to structural components of the ECM, where they are later activated by protease cleavage.7 Their regulatory actions are vital to the successful coordination of the many cellular and biochemical events in the healing cascade. The resulting wound bed, lacking attachment sites for migration, is unfavorable to keratinocytes leading to slow or absent wound closure. Additionally, fibroblasts and other vital cells in chronic wounds have been shown to be senescent, with decreased capacity to respond to growth factors and decreased ability for proliferating.1 Without the proliferation of fibroblasts, decreased amounts of collagen, fibronectin, and other vital matrix proteins are unable to flourish causing the wound healing cascade to stagnate.3Fibronectin is an essential glycoprotein of the extracellular matrix (ECM) with profound effects on the wound healing cascade. The fibrin clot (hemostasis), along with bound fibronectin, provide the structural framework for growth factors, proteases, and protease inhibitors.
Non-viable tissue deleteriously effects the wound environment by fostering bacterial growth and local tissue hypoxia. While immune cells secrete pro-inflammatory cytokines, the prolonged inflammation catalyzes neutrophils to produce copious amounts of reactive oxygen species (ROS).5 These chemical reactants serve to protect the body against impending infections however, in excess, can simultaneously damage the surrounding tissues.9 Infection augments ROS levels, thus promoting a hostile wound environment. Denervation is another critical factor that can delay the healing process in chronic wounds. Sensory nerves promote the release of essential neuropeptides (substance P) that serve as a chemotactic agent for inflammatory cellular recruitment.10 Consequently, denervation based wound etiologies (i.e. neuropathic ulcers, pressure ulcers) are subject to chronic inflammation and compromised healing.6The phagocytic and bactericidal properties of pro-inflammatory cells (macrophages and neutrophils) are diminished in a chronic wound environment.
Key macro-exogenous and endogenous factors that can delay wound healing include smoking/tobacco use, poor nutritional status, diabetes mellitus, obesity, certain pharmacologic agents, and obesity among others. Fortunately, cellular senescence instigated by a chronic inflammatory state is reversible by mitigating the factors that increase inflammation. In conjunction with routine wound bed preparation strategies, it is critical for clinicians to understand the mechanism of action of various advanced wound dressings in order to effectively modulate chronic wound environments.6Wound bed preparation is a precursor to the use of cellular and tissue based products.
WOUND BED PREPARATION
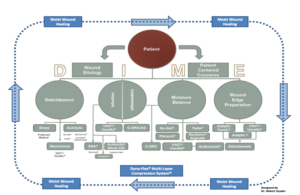
While various wound care guidelines exist and share certain elements of wound bed preparation, such as infection/inflammation management, and moisture balance etc, few clinical guidelines focus on patient centered concerns, despite the fact that outcomes are improved when these issues are addressed prior to initiating chronic wound treatment protocols. Comorbidities such as diabetes, obesity, arterial disease, smoking, and malnutrition, can affect the progression of wound healing. A multidisciplinary team approach should be initiated among caregivers and with their respective patients. Wound bed preparation is the essential framework for treatment pathways. It helps promote the acceleration of endogenous healing and facilitates the effectiveness of other therapeutic measures. This approach embodies a holistic, systematic, and multidisciplinary team approach that addresses patient centered concerns. The “DIME” algorithm is a well-established modality for proper wound bed preparation (Figure 2).11The mnemonic denotes debridement, infection control, moisture balance, and wound edge preparation. Active treatments engage several external mechanisms of action to eliminate necrotic (non-viable) tissue and or exudate from the wound bed via debridement and negative pressure wound therapy.7 Passive treatment promotes endogenous healing processes that remove the present obstacles to healing.7Some passive treatments include hydrogels (hydro-polymer dressings), hydrocolloid dressings, collagen-oxidized regenerated cellulose dressings, collagen-alginate dressings, cellulose-alginate dressings, non-adherent silicone dressings, and autolytic debridement.7Other treatment modalities include biologic skin substitutes (xerographs, allografts and dermal matrices) that support and promote wound closure.
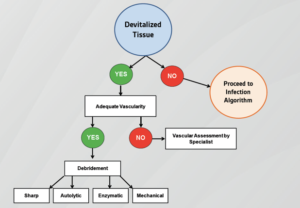
Debridement helps restore a viable wound base and fosters extra-cellular matrix protein assembly (Figure 3). The fundamental goal of debridement is to eliminate enough of the deleterious factors that will allow the wound to progress beyond the inflammatory stage toward healing.7 Debridement may also be of benefit by removing senescent cells that cause healing stagnation. Despite the fact that granulation tissue may appear “normal”, fibroblasts and other vital cells of wounds stuck in an inflammatory state may lose, to some degree, the ability to produce robust cytokines or the ability to manufacture collagen. Methods of debridement include sharp/surgical, autolytic, enzymatic, and biological (maggot larvae therapy).7 Surgical debridement will disrupt biofilm and remove the potential foci of infection. Wilcox et al have demonstrated higher healing rates observed when more frequent surgical debridement of diabetic foot ulcers was performed compared to ulcers not debrided as often.12The newly formed granulation tissue could have assisted in jump-starting the wound healing cascade. Autolytic debridement (saline gauze, alginates, hydrocolloids, etc.) will utilize the body’s endogenous healing capabilities and moisture to break down non-viable tissue. A key advantage of this form of debridement is that it does not damage the surrounding tissue (skin). This method can be used when the patient is not a candidate for sharp debridement or in the midst of severe infection. Enzymatic debridement modalities use chemical (exogenous) agents to break down non-viable/necrotic tissue. These topical agents (collagenases) disrupt or digest extracellular proteins.
Hydrogel based dressings composed of sodium alginate effectively debride necrotic tissue and fibrinous slough while maintaining a moist wound healing environment. Sodium carboxymethyl cellulose based dressings also promote autolytic debridement. It is important to note that debridement is essential to not only stimulate cellular communication but to also disrupt biofilm formation. However, it should be noted that during the physical elimination of biofilm, clinical and in vitro models have established that debridement opens a time-dependent window during which applied topical treatments can suppress biofilm reformation.
Infection is attributed to high bacterial loads that results in a prolonged inflammatory response. Treatment includes antimicrobial therapy (systemic & topical) with the goal of lowering bacterial bioburden and controlling inflammation. The increased bacterial burden may be confined to the superficial wound, deep compartments or around the surrounding tissue of wound margins. Previous studies have demonstrated that when bioburden reaches levels >105 bacteria per gram of tissue, infection can manifest.14 When bioburden levels >106, wound healing dysregulation promotes an inflammatory state.12 Previous studies have demonstrated that there is a homeostatic balance of protease activity in acute wounds, and high levels are found in stalled or chronic wound environments.15 However, for most clinicians, laboratory evaluation of protease activity is not widely accessible thus making clinical assessment paramount.
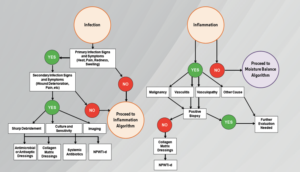
It is important to emphasize that in certain instances these high levels of bacterial bioburden can present without clinical signs of infection. Gardner et al assessed the reliability of clinical tools to evaluate the signs and symptoms of localized infections in chronic wounds.16 They established the ‘‘Clinical Signs and Symptoms Checklist,’’ which focuses on primary and secondary signs of infection to be reliable. Clinicians should diagnose infection based on the presence of at least two classic symptoms; inflammation or purulent secretions’. Incorporating a structured methodology to monitor and assess wound infections clinicians may improve their diagnostic and treatment trajectories (Figure 4). Additionally, a patient work-up should include a comprehensive series of lab studies (i.e. CBC, ESR, routine culture).
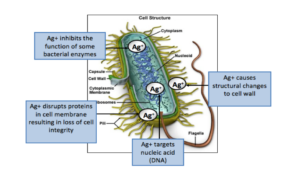
However, in order for wound dressings to effectively target these infectious agents, the biochemical network within bacterial cells must be inhibited (Figure 5). Electrons are one of the essential energy manufacturers within a bacterial cell. These molecules enable bacterial cells to undergo DNA replication, protein synthesis and cellular division. If these biochemical processes are obstructed, the bacterial cell will not survive. Silver, in its metallic form, is unreactive and does not have the ability to eradicate bacteria. In order to develop bactericidal capabilities, silver atoms must lose an electron and become positively charged silver ions. An advanced wound dressing with Ag OXYSALTS™ Technology is particularly effective because it is designed with three electrons missing (Ag3+), compared with other silver dressings that are only missing one electron (Ag1+). Thomason et al, presented in vivo and in vitro data that Ag OXYSALTS™ Technology wound dressings do not cause cytotoxicity.17 Increased reduction potentials allow the dressing to pull more electrons from bacteria to disrupt their function faster. Miller et al conducted an in vitro study and found that Ag OXYSALTS™ Technology dressings gave a log 7 reduction for both 24-hour and 72-hour biofilms.18 Infection is a major inhibitor of wound healing thus utilizing a multi-modal approach is critical in order to promote optimal patient outcomes.
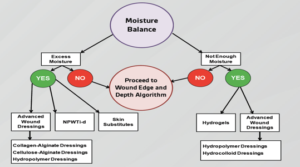
Moisture balance may describe wound environments from desiccation of tissue to excessive fluid leading to maceration and non-viable tissue. Biofilms have the ability
to permeate and reside throughout wound environments by feeding off fibrin slough. It is thought that biofilms delay wound healing by creating an unremitting hyper-inflammatory state by utilizing exudate as a fuel source, consequently, damaging host tissues. Interventions may include compression (when feasible) and various dressings that address the chronic wound ecosystem. Compression therapy is an essential treatment modality for venous leg ulcerations (VLUs). Previous studies have demonstrated that compression therapy facilitates faster healing of VLUs compared with no compression.19 Other dressing modalities include films, hydrogels, hydrocolloids, alginates, foams, and specialty absorbent products with usage dictated by the clinical picture (Figure 6). The main goal of moisture balance is to restore cellular communication and to avoid maceration of the wound bed and surrounding healthy tissue.
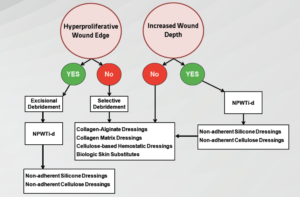
Edge preparation corrects non-advancing or undermined tissue predicated on non-migration of keratinocytes or senescent cells (Figure 7). A rolled or “cliff-like” wound edge also known as an epibole may be an accurate indication of inadequate wound bed preparation.20 Epiboles manifest when the epidermal margins of a wound are unable to migrate across a firm and level granulation base.16 Epidermal margins fail to migrate due to hypoxia, infection, shear, tension, desiccation, dressing trauma, hyperkeratosis, and callus at the wound margin, as well as a wound bed that is fibrinous, deficient in adhesion proteins, or is highly proteolytic obstructing cellular communication.16 The edge of the wound will not re-epithelialize unless the wound bed is well prepared. Interventions include debridement (to eradicate epiboles), biologic skin substitutes, topical growth factors, and skin grafts among other adjunctive therapies. A moist wound bed will promote vital cells to migrate and growth factors to help facilitate wound edge contraction.
CONCLUSION
Chronic wounds need a methodological preparation prior to therapeutic treatment plans. Assessment should include a comprehensive evaluation of the patient’s condition with key factors that include a detailed history and physical, laboratory screening, nutritional evaluation and vascular assessment. The DIME concept is a well-established, evidence-based principle of wound bed preparation. It involves effective debridement strategies, managing infection and persistent inflammation, moisture balance, and optimizing the wound edge.
After patient-centered concerns have been addressed and the wound bed has been sufficiently prepared, a treatment plan can be determined based on wound specific criteria. While biofilms are well-established to be an impediment to wound healing, the exact mechanisms remain a subject of continued research. There are currently no recognized clinical signs of biofilms in wounds that are readily available. However, the DIME multimodal approach should cause physical, chemical disruption and prevention of microbial cell attachment that could ultimately form a robust biofilm community.
Selection of the right dressing should be contingent on the etiology of the wound, amount of exudate and bioburden and the presence or absence of pain. Patients that are not responding to standard care should be treated with advanced therapies that may provide better outcomes and be more cost effective in the long run
References
1.Snyder RJ, Driver V, Fife CE et al. Using a diagnostic tool to identify elevated protease activity levels in chronic and stalled wounds: a consensus panel discussion. Ostomy Wound Manage 2011;57(12):36-46.
2.Herrick SE, Sloan P, McGurk M, Freak L, McCollum CN, Ferguson MW. Sequential changes in histologic pattern and extracellular matrix deposition during the healing of chronic venous ulcers. Am J Pathol 1992;141(5):1085-1095.
3.Lazaro JL, Izzo V, Meaume S, Davies AH, Lobmann R, Uccioli L. Elevated levels of matrix metalloproteinases and chronic wound healing: an updated review of clinical evidence. J Wound Care 2016;25(5):277-287. doi:10.12968/jowc.2016.25.5.277.
4.Suleman L. Extracellular Bacterial Proteases in Chronic Wounds: A Potential Therapeutic Target? Adv Wound Care 2016;5(10):455-463. doi:10.1089/wound.2015.0673.
5.Cullen B, Smith R, Mcculloch E, Silcock D, Morrison L. Mechanism of action of PROMOGRAN, a protease modulating matrix, for the treatment of diabetic foot ulcers. Wound Repair Regen 2002;10(1):16-25.
6.Falanga V. Growth factors and wound healing. Dermatol Clin 1993;11(4):667-675.
7.Lindley LE, Stojadinovic O, Pastar I, Tomic-Canic M. Biology and Biomarkers for Wound Healing. Plast Reconstr Surg 2016;138(3 Suppl):18S-28S. doi:10.1097/PRS.0000000000002682.
8.Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen 2011;19(2):134-148.
9.Sen CK. Wound healing essentials: let there be oxygen. Wound Repair Regen 2009;17(1):1-18. doi:10.1111/j.1524-475X.2008.00436.x.
10.Bryant RA, Nix DP. Acute & Chronic Wounds: Current Management Concepts. 5th ed. St. Louis, MO: Elsevier Inc.; 2016.
11.Snyder RJ, Fife C, Moore Z. Components and Quality Measures of DIME (Devitalized Tissue, Infection/Inflammation, Moisture Balance, and Edge Preparation) in Wound Care. Adv Skin Wound Care 2016;29(5):205-215. doi:10.1097/01.ASW.0000482354.01988.b4.
12.Wilcox JR, Carter MJ, Covington S. Frequency of debridements and time to heal: a retrospective cohort study of 312 744 wounds. JAMA Dermatology 2013;149(9):1050-1058. doi:10.1001/jamadermatol.2013.4960.
13.Wolcott RD, Rumbaugh KP, James G et al. Biofilm maturity studies indicate sharp debridement opens a time- dependent therapeutic window. J Wound Care 2010;19(8):320-328.
14.Robson MC. Wound infection. A failure of wound healing caused by an imbalance of bacteria. Surg Clin North Am 1997;77(3):637-650.
15.Trengove NJ, Stacey MC, MacAuley S et al. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen 1999;7(6):442-452.
16.Gardner SE, Frantz RA, Troia C et al. A tool to assess clinical signs and symptoms of localized infection in chronic wounds: development and reliability. Ostomy Wound Manage 2001;47(1):40-47.
17.Thomason HA, Lovett JM, Spina CJ, Stephenson C, McBain AJ, Hardman MJ. Silver oxysalts promote cutaneous wound healing independent of infection. Wound Repair Regen 2018. doi:10.1111/wrr.12627.
18.Miller S, Lemire J, Brabu A et al. Antimicrobial silver in medical devices: composition and efficacy [abstract]. Presented at SAWC Spring 2013, May 1-5, 2013, Denver, CO 2013.
19.Nair B. Compression therapy for venous leg ulcers. Indian Dermatology Online Journal 2014;5(3):378-382. doi:10.4103/2229-5178.137822.
20.Schultz G, Dowsett C. Technology Update: Wound bed preparation revisited. Wounds International 2012;3(1):25-29.