Dr. Barrett is Program Director of the Center for Wound Healing at Crozer Chester Medical Center, Chester, PA. He is a surgically trained podiatrist with 15 years of experience developing and managing centers of excellence for amputation prevention, wound management and hyperbaric medicine. He is an invited speaker and presenter at national and international wound care conferences; sharing insight and experiences using established and emerging wound care therapies to improve outcomes.
Barrett_Current Dialogues in Wound Management_2017_Volume 3_Issue 2
NOTE: As with any case study, the results and outcomes should not be interpreted as a guarantee or warranty of similar results. Individual results may vary depending on the patient’s circumstances and condition.
The statistics may no longer shock those of us who have devoted our lives to treating patients with chronic wounds but they can’t be ignored:
•Approximately 29.1 million people or 9.3% of the population have diabetes1
•Five-year mortality rates for new-onset diabetic ulcerations are higher than those of several types of cancer including breast, prostate, colon and Hodgkin’s disease2
•Each year, more than 2.5 million people in the United States develop pressure ulcers3
•Venous leg ulcers affect 2.2 million Americans annually4
•Chronic wounds affect 6.5 million patients in the United States with a cost estimated in excess of $25 billion annually
An increasingly sedentary lifestyle in recent decades has been linked to a rise in cardiovascular disease, type 2 diabetes, obesity, and some cancers.6, 7 With an aging population worldwide, we have seen a corresponding rise in age-associated diseases, including wound healing disorders.8 In addition to the previously reported economic burden and high mortality and morbidity associated with these disorders, the global market for advanced wound care products is expected to reach
$14.9 billion by 2021.
Wound care has not only become big business, it has also become more sophisticated. With the advances made in understanding the science of wound healing, in addition to the flood of new biologics, dressing selection, ICD-10 and procedure bundling, the modern wound care clinic can be a daunting and confusing place for the untrained clinician. Based on my experience, that lack of formalized wound care training may result in inappropriate use of resources, increased costs and poor outcomes. It’s not difficult to understand why the CMS and many private payors deny coverage for untested and/or over-utilized wound care modalities and resort to aggressive cost-cutting measures. As healthcare moves from a fee-for-service payor system to one that rewards quality and value, maintaining a profitable, outcome-driven, evidence-based practice will be a significant challenge for many.
BACK TO BASICS: WOUND BED PREPARATION
Before we can discuss the details of any wound care product, we must first understand chronic wound healing, why a given product’s mechanism of action would be beneficial and how we can best utilize the product in our treatment plan. The integrated concept of wound bed preparation and the acronym TIME was introduced in 2003 by a group of physicians, nurses and scientists. TIME is a systematic approach to wound management and is based on intervention in four key clinical areas (T-tissue, I-infection/inflammation, M-moisture balance, E-edge of wound/epithelial migration). The goal of TIME is to remove the barriers to healing and re-establish an optimal wound bed that will then respond effectively to a given therapeutic regimen.10This regimen could include NPWT but for the outpatient wound clinic, there may be an excuse for underutilization.
 Figure 3: February 11, 2016 & Figure 4: March 3, 2016
Figure 3: February 11, 2016 & Figure 4: March 3, 2016
TRADITIONAL NPWT: DOES ONE SIZE FIT ALL?
Topical negative pressure wound therapy is now widely accepted as an effective method for managing both acute and chronic wounds with numerous publications detailing its mechanism of action and supporting its use.11- 14 The first commercialized device, Vacuum-Assisted Closure (V.A.C.® Therapy, Kinetic Concepts, Inc. [KCI], an Acelity Company, San Antonio, Texas) was introduced in the United States in the late 1990s. Many of the typical wounds encountered in an outpatient wound clinic are much smaller than the large, complex wounds that the powered NPWT systems were designed for. These smaller wounds have significantly lower exudate levels and do not require the capacity and corresponding bulk of electronically powered NPWT.
THE SNAP™ THERAPY SYSTEM: SMART NEGATIVE PRESSURE
If you had to design an NPWT system ideally suited for the ambulatory wound care patient it would be lightweight, silent and completely portable, and could be worn discreetly under clothing when in use. If your system also had to appeal to the outpatient wound clinic treating the patient it would be inexpensive, completely disposable and available “off-the shelf” when you need it, avoiding the need for a time consuming durable medical equipment (DME) rental application and delivery delay. This technology exists as the SNAP™ Therapy System (Kinetic Concepts, Inc. [KCI], an Acelity Company, San Antonio, Texas). Two randomized-controlled trials provide evidence supporting the clinical effectiveness of the SNAP™ Therapy System when compared to the KCI V.A.C.® Therapy.15,16The SNAP™ Therapy System consists of five basic elements: the vacuum/exudate cartridge, activation/reset key, hydrocolloid dressing with integrated micro-port, cut-to-length tubing and open cell foam wound interface. The cartridge requires no electricity or battery recharging as vacuum is maintained by a silent, proprietary spring mechanism. As a result, patients are not prevented from continuing to work while using the device. The SNAP™ Therapy System can be worn discreetly under clothing and allows patients to remain social, active and maintain their quality of life. For the wound care clinic, the ability to purchase the SNAP™ Dressing Kits and keep them in stock for immediate use when they are most needed is a benefit for both the patient and the nursing staff. The SNAP™ Therapy System is not DME and as such, each application is billed directly to the patient’s insurance using ® (CPT is a trademark of the American Medical Association.) codes 97607/97608. For even greater continuity of care, as of January 1, 2017, eligible Medicare beneficiaries can now have a home health agency apply the SNAP™ Therapy System disposable negative pressure dressings if ordered by their physician or non-physician provider (NPP).
WOUND BED PREPARATION AND THE SNAP™ THERAPY SYSTEM: A PERFECT UNION
If we follow the guidance of TIMEeffectively we would expect to have a clean, viable wound bed, a canvas upon which we can apply evidence-based interventions to achieve closure. But if we look more closely at each individual element of TIME, you will see a potential role for NPWT in your treatment plan. Management of biofilm in chronic wounds is rapidly becoming a primary objective of wound care. In light of the limitations of current microbiological testing, it is speculated that the true prevalence of biofilm approaches 100%.19 Necrotic, non-viable tissue, biofilm and excessive protease activity (T, I) all contribute to wound inflammation, the proverbial “stalled” wound. Poorly managed wound exudate (M) leads to maceration and peri-wound damage. The result (E) is a non-migrating wound edge. The TIME framework’s recommended initial interventions (debridement, wound cleansing, moisture control) offer a temporary solution, as biofilm and inflammation will rapidly return within 24 hours.
In the outpatient wound clinic, the decision on how to prevent our prepared wound bed from regressing must take into account several factors: clinical effectiveness, cost, reimbursement and most importantly, our patient’s needs. Let’s look at the clinical benefits of NPWT: removing infectious material while being cost-effective, positively reimbursed and patient-centered. Because the SNAP™ Therapy System is “off-the-shelf”, NPWT can be utilized immediately following wound bed preparation when it is most needed. Our individualized treatment plans include protease modulating dressings, high-level compression (Case 1) and biologics (Case 2) for wound bed preparation. Our wound bed preparation is successful and our interventions move the wound to healing.
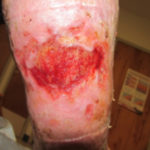
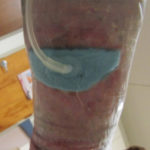
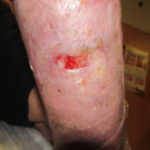
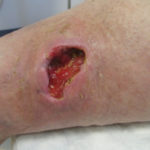
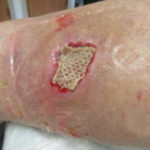
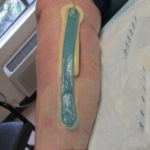
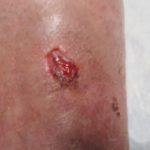
In examining our first case, we present a venous leg ulcer with poorly managed exudate, inflammation, and peri-wound skin damage with dull, thin granulation tissue base (Figure 1). Our treatment strategy followed the TIME principles of wound management and included the use of a protease-modulating extracellular matrix (ECM) collagen, four-layer compression (Figure 3) and the SNAP™ Therapy System (Figure 2). At follow up, the patient’s wound demonstrates reduced inflammation, improved wound bed perfusion, restored peri-wound health (Figure 3) and advanced epithelial migration (Figure 4)
Case 1
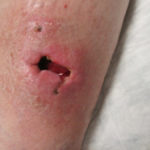
Our second case involves a dog bite injury to the right anterior leg. The wound shows significant undermining (Figure 6) which required operative resection (Figure 7) in order for the optimal preparation of the wound for further treatment. Our ensuing treatment approach to this wound involved the utilization of a cryopreserved human skin allograft (Figure 8) under the SNAP™ Therapy System (Figure 9) with eventual closure utilizing grafts harvested with the CELLUTOME™ Epidermal Harvesting System (Figure 10).
Case 2
In conclusion, our experience demonstrates that the SNAP™ Therapy System is a truly unique and versatile method of utilizing the multi-tasking abilities of NPWT in the wound clinic where it is most needed. The system is low-cost, equally as effective as electrically powered NPWT15, positively reimbursed and designed for the smaller, low-exudate chronic wound that is typically encountered in outpatient and home care settings. In our center’s opinion, the SNAP™ Therapy System is the future of outpatient NPWT available now.
References
1.Centers for Disease Control and Prevention. National Diabetes Statistics Report 2014. https:// www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Updated May 15, 2015.
2. Robbins JM, Stauss G, Aron D, Long J, Kuba J, Kaplan Y. Mortality rates and diabetic foot ulcers: is it time to communicate mortality risk to patients with diabetic foot ulceration? J Am Podiatr Med Assoc. 2008;98(6):489-93. https://www.ncbi.nlm.nih.gov/pubmed/19017860.
3. Berlowitz D ,VanDeusen Lukas C, Parker V, et al. Preventing Pressure Ulcers in Hospitals. https://www.ahrq.gov/sites/default/files/publications/files/putoolkit.pdf.
4. Alavi A, Sibbald RG, Phillips TJ, et al. What’s new: Management of venous leg ulcers: Approach to venous leg ulcers. J Am Acad Dermatol. 2016;74(4):627-40; quiz 641-2. doi:10.1016/j.jaad.2014.10.048.
5. Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763-71. doi:10.1111/j.1524-475X.2009.00543.x.
6. Neville O, Sparling PB, Healy GN, Dunstan DW, Matthews CE. Sedentary Behavior: Emerging Evidence for a New Health Risk. Mayo Clin Proc. 2010; 85(12): 1138–41. doi:10.4065/mcp .2010.0444.
7. Buchner DM, Bishop J, Brown DR, et al. 2008 Physical Activity Guidelines for Americans. Health.gov. https://health.gov/paguidelines/pdf/paguide.pdf. Published October 2008. Accessed August 24, 2010.
8. Makrantonaki E, Wlaschek M, Scharffetter-Kochanek K. Pathogenesis of wound healing disorders in the elderly. J Dtsch Dermatol Ges. 2017;15(3):255-75. doi:10.1111/ddg.13199.
9. Advanced Wound Care Market by Type (Dressings, Therapy Devices, Active Wound Care), Application (Surgical Wounds, Ulcers), End User (In-Patient Facility, Out-Patient Facility), and Geography – Global Forecast to 2020. Markets and Markets Web site. http://www.marketsandmarkets.com/Market-Reports/advanced-wound-care-market-88705076.html. Published October 2015.
10. Schultz GS, Sibbald RG, Falanga V, et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen. 2003;11(suppl 1):1S-28S. https:// www.ncbi.nlm.nih.gov/pubmed/12654015.
11. Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997;38(6):563-76; discussion 577. https://www.ncbi.nlm.nih.gov/ pubmed/9188971.
12. Orgill DP, Bayer LR. Negative pressure wound therapy: past, present and future. Int Wound J. 2013;10 (Suppl 1):15-9. doi:10.1111/iwj.12170.
13. Saxena V, Hwang CW, Huang S, Eichbaum Q, Ingber D, Orgill DP. Vacuum-assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg. 2004;114(5):1086-96; discussion 1097-8. https://www.ncbi.nlm.nih.gov/pubmed/15457017.
14. Armstrong DG, Lavery LA; Diabetic Foot Study Consortium. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet. 2005;366(9498) :1704-10. doi:10.1016/S0140-6736(05)67695-7.
15. Armstrong DG, Marston WA, Reyzelman AM, Kirsner RS. Comparison of negative pressure wound therapy with an ultraportable mechanically powered device vs. traditional electrically powered device for the treatment of chronic lower extremity ulcers: A multicenter randomized-controlled trial. Wound Repair Regen.2011;19(2):173-80. doi:10.1111/j.1524-475X.2010 .00658.x.
16. Marston WA, Armstrong DG, Reyzelman AM, Kirsner RS. A Multicenter Randomized Controlled Trial Comparing Treatment of Venous Leg Ulcers Using Mechanically Versus Electrically Powered Negative Pressure Wound Therapy. Adv Wound Care. 2015;4(2):75-82. doi:10.1089/wound .2014.0575.
17. MLN Matters. Centers for Medicare & Medicaid Services Web site. https://www.cms.gov/outreach-and-education/medicare-learning-network-mln/mlnmattersarticles/downloads/mm9898.pdf.
18. Bjarnsholt T, Cooper R, Fletcher J, et al. MANAGEMENT OF BIOFILM The role of biofilm in delayed wound healing Biofilm management in practice How is research advancing the understanding of biofilm. World Union of Wound Healing Societies Web site. http://www.wuwhs2016.com/files/WUWHS_Biofilms_web.pdf. Published 2016.